Novel Therapies for Metastatic Triple-Negative Breast Cancer: Spotlight on Immunotherapy and Antibody-Drug Conjugates
This review article published in the journal ONCOLOGY® looks at the successful clinical development of immunotherapies, PARP inhibitors, and antibody-drug conjugates for the management of metastatic triple-negative breast cancer and how these have improved the survival outcome of patients. Over the coming years, therapeutic developments in precision medicine will likely change the treatment landscape and might make the current definition of triple-negative breast cancer as a disease that is estrogen receptor, progesterone receptor, and HER2 negative obsolete.
Nagayama is from the Department of Surgery (Breast Group) at the Keio University School of Medicine in Tokyo, Japan.

Vidula is from the Massachusetts General Hospital Cancer Center and Harvard Medical School in Boston, MA.

Bardia is from the Massachusetts General Hospital Cancer Center and Harvard Medical School in Boston, MA.

ABSTRACT
Background: Triple-negative breast cancer (TNBC) is a biologically heterogeneous disease that is often associated with worse outcomes compared with other subtypes such as hormone receptor–positive tumors and HER2-positive tumors. While chemotherapy remains the mainstay of standard therapy for metastatic TNBC (mTNBC), several novel treatments have been developed over the past few years. In this review article, we review the major developments in the management of patients with mTNBC.
Summary: The combination of chemotherapy and immunotherapy is a potential therapeutic option for PD-L1–positive mTNBC, as the FDA recently approved atezolizumab (Tecentriq) and pembrolizumab (Keytruda) in combination with chemotherapy. Also, 2 targeted therapies—olaparib (Lynparza) and talazoparib (Talzenna)—are FDA approved for the management of mTNBC with germline BRCA mutations, and sacituzumab govitecan, an anti-Trop2 antibody-drug conjugate (ADC), was recently approved for previously treated mTNBC. A number of promising therapies are on the horizon, including AKT inhibitors for PI3K-altered TNBC as well as other ADCs.
Key Message: The successful clinical development of immunotherapies, PARP inhibitors, and ADCs for the management of mTNBC has improved the survival outcome of patients. Over the coming years, the therapeutic developments in precision medicine will likely change the mTNBC landscape, and might make the current definition of TNBC as breast cancer that is estrogen receptor negative, progesterone receptor negative, and HER2 negative obsolete.
Introduction
Triple-negative breast cancer (TNBC) is a heterogenous disease that essentially is a diagnosis of receptor exclusion, referring to tumors that lack hormone receptors and lack of HER2 amplification. Clinically, patients with TNBC, as compared with other breast cancer subtypes, have a higher risk of recurrence and poor prognosis.1 Better treatments for TNBC represent a major unmet clinical need in the field of breast oncology, and over the past few years, a number of attempts have been made to change the natural history of this biologically aggressive disease.
One of the major breakthroughs for TNBC has come from a better understanding of the molecular subtypes of TNBC. Recent genomic, transcriptomic, proteomic, and epigenomic studies have shed light on the multilayered heterogeneity of TNBC tumors (Table 1). In one of the first descriptions of the heterogeneity of TNBC, Lehmann et al reported that there are at least 6 subtypes of TNBC: basal-like 1 (BL1), basal-like 2 (BL2), immunomodulatory (IM), mesenchymal (M), mesenchymal stem-like, and luminal androgen receptor (LAR), with distinct gene expression profiles.2 Each of these molecular subtypes displays different but overlapping clinicopathological characteristics, heterogenous mutational profiles, and genomic instability.3 This classification was later refined to 4 subtypes—BL1, BL2, M, and LAR—by excluding the contribution from tumor-infiltrating lymphocytes (TILs) and stromal cells.4 A recent study of whole-genome sequencing of TNBC tumors also confirmed that 59% were predicted to be homologous recombination repair deficiency (HRD) high, mainly by BRCA1/2 deficiency, and HRD-high patients demonstrated better prognosis whereas HRD-low patients, some with mismatch repair deficiency and PIK3CA/AKT1 pathway alterations, demonstrated worse prognosis.5 On the protein level, quantitative analysis of TNBC cell lines and breast tumors again showed diverse expression of signaling and biological pathways.6 Furthermore, some of the hormone receptor–positive tumors can lose the hormone receptors under pressure from endocrine therapy and evolve into acquired TNBC with a distinct genomic profile.7,8 Collectively, TNBC tumors display considerable intratumoral heterogeneity, highlighting the potential value of single-cell sequencing over bulk RNA sequencing in understanding the biology of TNBC.9
TABLE 1. Key Studies Reporting Genetic and Transcriptomic Classifications in Early Triple-Negative Breast Cancer
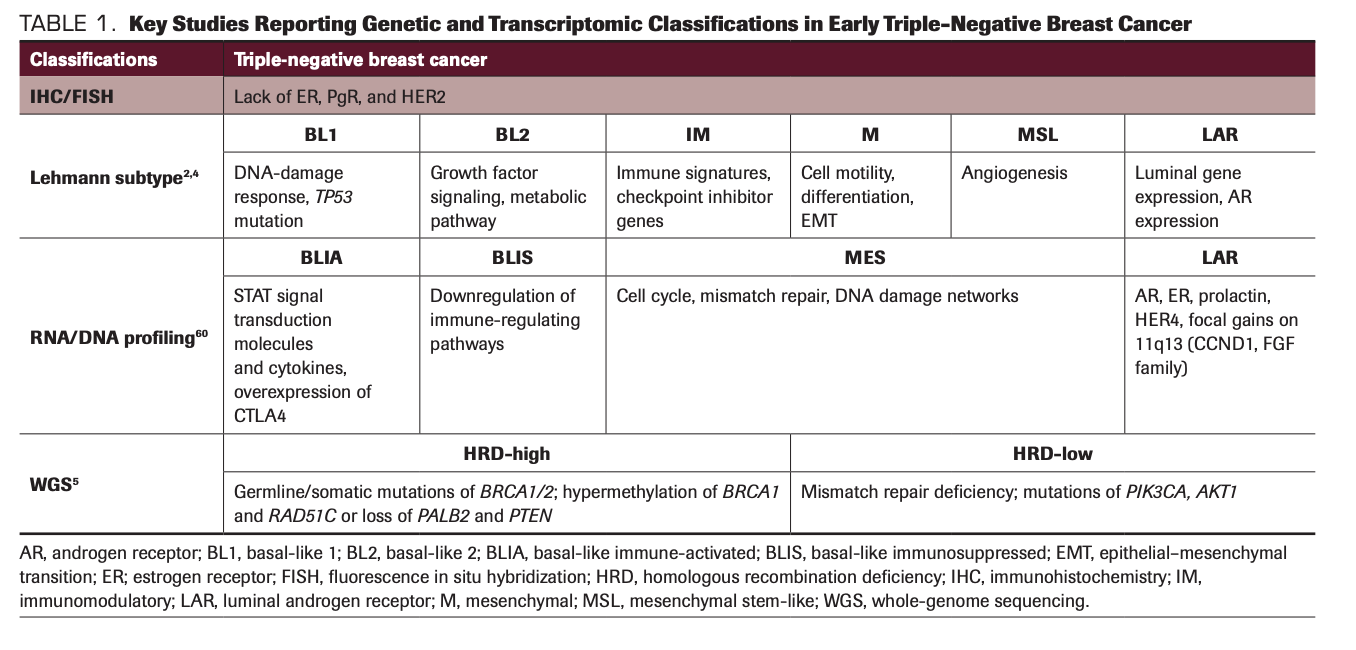
In terms of treatment targets in TNBC tumors, traditional chemotherapies, such as anthracycline, taxanes, and platinum agents, have been the mainstay of early-line management of mTNBC. Apart from cytotoxic chemotherapy, other treatment strategies and actionable targets such as PARP inhibition, immunotherapy, AKT inhibition, and antibody-drug conjugates (ADCs) in mTNBC are quickly drawing attention. In this review article, we discuss the major developments of targeted therapies and immunotherapies in the management of patients with mTNBC.
PARP Inhibitors for BRCA-Mutated TNBC
The DNA damage response process involves sensing and responding to DNA damage, mediating DNA repair, cell cycle regulation, replication stress response, and apoptosis.10 The deficiency of DNA damage response harbors genomic instability in cells, but it also provides therapeutic targets, particularly to DNA-damage agents. Carriers of deleterious heterozygous germline mutations in the BRCA1 and BRCA2 genes demonstrate the deficiency of homologous recombination repair, a conservative process of repairing double strand DNA breaks. PARP inhibitors induce apoptosis in BRCA-deficient tumor cells by mediating stalled replication forks and double-strand DNA breaks.11 For patients with germline BRCA1/2 mutations, PARP inhibitors, including olaparib (Lynparza) and talazoparib (Talzenna), are FDA approved as targeted therapies.
The efficacy and safety of olaparib was demonstrated in the randomized, open-label, phase 3 OlympiAD trial (NCT02000622) conducted in patients with metastatic HER2-negative breast cancer with germline BRCA mutations. Between April 2014 and November 2015, 205 participants were assigned to receive olaparib and 97 to receive standard therapy with single-agent chemotherapy of physician’s choice (capecitabine, eribulin, or vinorelbine). The results demonstrated a significant improvement in median progression-free survival (PFS) with olaparib (7.0 vs 4.2 months; HR, 0.58; P <.001) with fewer grade 3 and above adverse events (AEs) in the olaparib arm. The response rate was 59.9% in the olaparib group and 28.8% in the standard-therapy group.12 The final overall survival (OS) analysis demonstrated no statistically significant improvement (19.3 vs 17.1 months; HR, 0.90; P = .513).13 In the TNBC subgroup, PFS favored olaparib (HR, 0.43, 95% CI, 0.29-0.63) but the median OS was not statistically different between the 2 groups (17.4 vs 14.9 months; HR, 0.93; P = .51). Similarly, another PARP inhibitor, talazoparib, was investigated in a randomized, open-label, phase 3 EMBRACA trial (NCT01945775) in patients with advanced breast cancer and germline BRCA1/2 mutations.14 Between October 2013 and April 2017, 287 patients were assigned to receive talazoparib and 144 to standard therapy (capecitabine, eribulin, gemcitabine, or vinorelbine). Median PFS was significantly improved in the talazoparib group compared with the standard therapy group (8.6 vs 5.6 months; HR, 0.54; P <.001) with manageable toxicities.15 Again, the OS analysis demonstrated no statistically significant improvement (19.3 vs 19.5 months; HR, 0.848; P = .17) in the entire cohort.16 In TNBC subgroup (130 patients in the talazoparib arm and 60 patients in the standard-therapy arm), PFS favored talazoparib (5.8 vs 2.9 months; HR, 0.60; 95% CI, 0.41-0.87; P = .0058).16
Putting together these data, PARP inhibitors for patients with germline BRCA1/2 mutation improved clinically meaningful PFS with manageable toxicity. The potential utility of PARP inhibitors in TNBC is being explored in patients with early-stage breast cancer in the neoadjuvant17 and adjuvant settings,18 in overcoming resistance by combination therapy,19 and in wider patient selection with HRD phenotype beyond germline BRCA mutations.20 Notably, a single agent, olaparib, achieved an 82% response rate in 11 patients with germline PALB2 mutation in the recent proof-of-principle study.21 The comparison of efficacy and safety of a PARP inhibitor vs platinum-based treatment in patients with TNBC and germline BRCA1/2 mutation has not yet been done.
Immunotherapy for mTNBC
In the past decade, considerable evidence has been uncovered showing that tumors acquire an immune escape system to facilitate tumor growth by usurping the PD-1/PD-L1 axis between TILs and tumor cells.22 Also, inhibiting PD-L1 can induce antitumor activity.23
The pivotal IMpassion130 (NCT02425891) phase 3 trial investigated a selective PD-L1 inhibitor, atezolizumab (Tecentriq), plus nab-paclitaxel (Abraxane) vs placebo plus nab-paclitaxel in patients with mTNBC. From June 2015 through May 2017, a total of 451 patients were randomized on the study. Patients receiving atezolizumab plus nab-paclitaxel had a statistically significant but clinically moderate improvement in PFS in the PD-L1–positive subgroup (7.5 vs 5.0 months; HR, 0.62; 95% CI, 0.49-0.78; P < .001).24 However, a more impressive and significant improvement in OS (25.4 vs 17.9 months; HR, 0.67; 95% CI, 0.53-0.86) was demonstrated in the final analysis.25,26 This led to the FDA approval of atezolizumab in combination with nab-paclitaxel as first-line therapy for PD-L1–positive (defined as ≥ 1% in immune cells by the SP142 Ventana assay) mTNBC in 2019.
In contrast to the results of IMpassion130, the phase 3 IMpassion131 study (NCT03125902) evaluated atezolizumab in combination with paclitaxel compared with placebo plus paclitaxel and failed to demonstrate an improvement in PFS and OS.26 This study enrolled and randomized 651 participants in a 2:1 ratio to receive atezolizumab plus paclitaxel or placebo plus paclitaxel. The primary end point, PFS in the PD-L1–positive population, was not statistically different between the 2 arms (6.0 vs 5.7 months; HR, 0.82; P = .20). Further research is needed to better understand these results, and how the difference of chemotherapy partner, and the impact of steroids in modulating a response to immunotherapy, may have contributed to the study’s results. More research is also needed to understand how to optimize PD-L1 positivity evaluation to select patients who may benefit from immunotherapy.27
As per the FDA label, the atezolizumab approval is for “adult patients with unresectable locally advanced or metastatic TNBC whose tumors express PD-L1 (PD-L1 stained tumor-infiltrating immune cells of any intensity covering ≥ 1% of the tumor area), as determined by an FDA-approved test.” This is particularly important because there is considerable variation in the median PFS estimates with immunotherapy depending on the type of assay used.27 A post hoc analysis evaluated PD-L1 status using 3 assays: Ventana SP142, SP263 immunohistochemistry (IHC) assay (positivity defined as 1% or more PD-L1 stained immune cells), or the Dako PD-L1 IHC 22C3 assay (positivity defined as 1 or more combined proportion score [CPS]). Patients in the SP142 PD-L1–positive population achieved the greatest clinical benefit from atezolizumab plus nab-paclitaxel. Thus, PD-L1 expression as a predictive biomarker needs to be assessed cautiously due to its variability with measurement assays and positivity thresholds both within and across tumor types.28
The optimal criteria and assay of PD-L1 positivity continued to be important considerations in KEYNOTE-355, the study of another immune checkpoint inhibitor, pembrolizumab (Keytruda). The phase 3 KEYNOTE-355 trial (NCT02819518) investigated pembrolizumab with chemotherapy (investigator’s choice of either nab-paclitaxel, paclitaxel, or gemcitabine/carboplatin) compared with placebo and chemotherapy for patients with previously untreated, advanced or metastatic TNBC whose tumors expressed PD-L1, defined by a CPS of 10 or greater, a different assay from the SP142/Ventana assay utilized in the IMpassion130 trial.29 The study enrolled 847 patients and demonstrated a statistically significant improvement in PFS with pembrolizumab and chemotherapy compared with chemotherapy alone (9.7 vs 5.6 months; HR, 0.65; P = .0012) in the population with CPS 10 or greater.30 The study results led to the regulatory approval of pembrolizumab for patients with PD-L1–positive mTNBC, in combination with various chemotherapy partners, in November 2020.
Targeting the PIK3CA/AKT/PTEN Pathway
The PIK3CA/AKT signaling pathway is often activated through activation mutations in PIK3CA or AKT1 or alterations in PTEN31,32; also, approximately half of TNBC tumors have deficient expression of tumor suppressor PTEN,3 which is associated with AKT pathway activation. These findings have led to considerable therapeutic interest in evaluating AKT inhibitors, particularly in combination with chemotherapy as well as immunotherapy.
The LOTUS trial (NCT02162719), a randomized, double-blind, phase 2 trial, evaluated the efficacy of a selective oral ATP-competitive small molecule AKT inhibitor, ipatasertib, as first-line therapy in combination with paclitaxel, vs placebo and paclitaxel in patients with mTNBC.33 Between September 2014 and February 2016, 62 patients each were assigned to ipatasertib plus paclitaxel and to placebo plus paclitaxel. The addition of ipatasertib to paclitaxel was associated with a longer median PFS in the intention-to-treat population (6.2 vs 4.9 months; HR, 0.60; 95% CI, 0.37-0.98; P = .037). Although the PFS in 48 patients with PTEN-low tumors assessed by IHC failed to demonstrate a statistically significant improvement with ipatasertib vs placebo, 42 patients with genetic PTEN-inactivating alterations or PIK3CA/AKT1-activating mutations demonstrated a PFS improvement with ipatasertib (9.0 vs 4.9 months; nonstratified HR, 0.44; 95% CI, 0.20-0.99; P = .041).33 The median OS was numerically longer in the ipatasertib arm compared with placebo (25.8 vs 16.9 months; HR, 0.80), but given the phase 2 design needed confirmation in a phase 3 trial.34
As follow-up to the LOTUS trial, the IPATunity130 phase 3 registration trial (NCT03337724) assigned 168 patients with advanced or metastatic TNBC with PIK3CA/AKT1/PTEN alterations to receive ipatasertib, and 87 patients to receive placebo, both combined with paclitaxel on days 1, 8, and 15 of 28-day cycles.35 Surprisingly, there was no difference in median PFS between the 2 groups (7.4 vs 6.1 months; HR, 1.02; 95% CI, 0.71-1.45; P = .9237) after 8.3 months of follow-up. Thus, the phase 3 trial failed to demonstrate the PFS benefit expected based on the results of the phase 2 LOTUS trial. The OS data are still immature, and a question that needs to be addressed is whether there may be a subpopulation among patients with PIK3CA/AKT1/PTEN–altered TNBC who may derive benefit from the drug. In addition, a pan-class I PI3K inhibitor, buparlisib, failed to improve the outcome. In the adaptive phase 2/3 BELLE-4 study (NCT01572727), 416 patients with HER2-negative locally advanced and metastatic breast cancer with no prior chemotherapy in the advanced setting were randomized 1:1 to receive either buparlisib plus paclitaxel or placebo plus paclitaxel.36 No PFS improvement occurred with buparlisib vs placebo in the entire cohort (8.9 vs 9.2 months, respectively; HR, 1.18) or PI3K pathway–activated cohort (9.1 vs 9.2 months, respectively; HR, 1.17). The results of this study also contrasted with the findings from the LOTUS trial. Capivasertib is another potent, highly selective oral small molecule that inhibits the isoforms of AKT1, AKT2, and AKT3.37,38 The PAKT trial (NCT02423603) is a double-blind, randomized phase 2 trial that enrolled patients with untreated mTNBC. Between May 2014 and June 2017, a total of 140 patients were randomly assigned to paclitaxel with either capivasertib or placebo in a 1:1 ratio. Similar to LOTUS, this study demonstrated an improvement in median PFS with capivasertib and paclitaxel compared with paclitaxel and placebo among 28 patients with genetic alterations of PI3CA, AKT1, PTEN (9.3 vs 3.7 months; HR, 0.30; 95% CI, 0.11-0.79; P = .01).39 The most frequent serious AE was diarrhea, experienced in 13.2% of the capivasertib cohort.
Overall, the best approach to incorporate an AKT inhibitor into the treatment of TNBC has yet to be established.40 More specifically, questions remain as to which PTEN alterations confer sufficient loss of function to be targeted as frameshift mutations.
Antibody-Drug Conjugates for mTNBC
Finally, ADCs have gained considerable momentum in the field of mTNBC. An ADC is composed of 3 well-defined components: a monoclonal antibody (mAb), a cytotoxic payload, and a linker that connects them. A cancer-specific antibody binds to an antigen on the cell surface, gets internalized, and releases its potent payload to take effect.41 Apart from the selective delivery of ADC, the mAb induces apoptosis by inhibiting signal transduction42 or by the bystander killing effect, in which free payload in the cytosol permeates the plasma membrane in the tumor microenvironment to impact cells, including those that do not express the target antigen.43
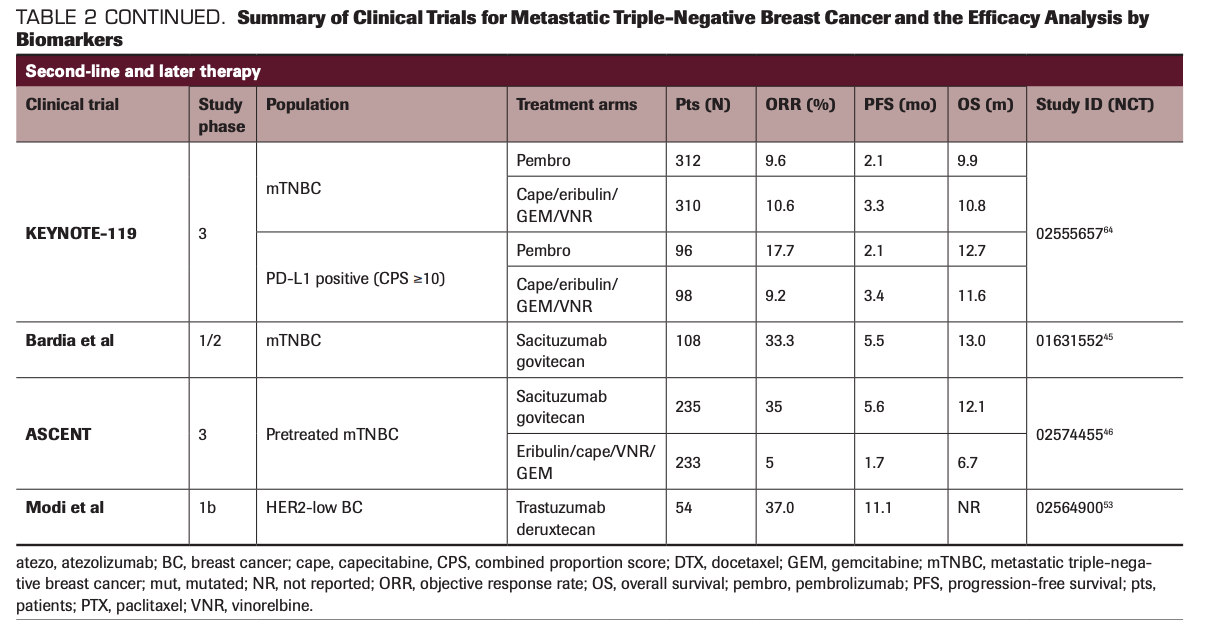
Sacituzumab govitecan (IMMU-132; Trodelvy) is an ADC that targets Trop-2, which is overexpressed in the majority of TNBC, with the antibody linked with SN-38, a topoisomerase-1 inhibitor.44 In a basket single-arm phase 1/2, multicenter trial enrolling patients with advanced epithelial cancers, sacituzumab govitecan demonstrated considerable single-agent activity among heavily pretreated patients with mTNBC.45 A total of 108 patients received sacituzumab
govitecan and the response rate was 33.3% (95% CI, 24.6%-43.1%) and the median PFS was 5.5 months (95% CI, 4.1-6.3). This result compared favorably with the historical data of response rates with chemotherapy, thus leading to the accelerated approval of sacituzumab govitecan by the FDA in April 2020. Shortly after this approval, results of the confirmatory phase 3 trial (ASCENT; NCT02574455) investigating sacituzumab govitecan vs chemotherapy of physician’s choice (eribulin, capecitabine, gemcitabine, or vinorelbine) in mTNBC were reported. This trial demonstrated a survival benefit from sacituzumab govitecan with a significantly improved median PFS (5.6 vs 1.7 months; HR, 0.41; P <.0001) and median OS (12.1 vs 6.7 months; HR, 0.48;
P <.0001) compared with chemotherapy of physician’s choice.46 Also, sacituzumab govitecan induced clinical benefit—improved PFS and OS—over physician’s choice of therapy in patients with mTNBC, irrespective of Trop-2 expression; however, greater efficacy was observed in those who had a medium or high Trop-2 score, according to data from an exploratory biomarker analysis of the phase 3 ASCENT trial that were presented during the 2020 San Antonio Breast Cancer Symposium. The most common any-grade AEs included nausea, neutropenia, diarrhea, fatigue, and anemia. Due to its compelling evidence of efficacy and no additional safety concerns, this trial was terminated early based on the recommendation by the independent data safety monitoring committee in March 2020. Collectively, these results demonstrate the promising potential of sacituzumab govitecan to impact the standard management of pretreated mTNBC.
Similarly, ladiratuzumab vedotin, an ADC targeting LIV-1, combined with a microtubule inhibitor, monomethyl auristatin E, as the toxic payload, has been developed for patients with relapsed/refractory TNBC.47 As a monotherapy, ladiratuzumab vedotin is being studied to determine its safety and tolerability profile in patients with LIV-1–positive advanced or metastatic breast cancer, including 51 patients with mTNBC, in a phase 1, open-label, dose-escalation study (NCT01969643). Preliminary results from this trial have shown an objective response rate (ORR) of 32% and clinical benefit rate (CBR) of 36% with good tolerability, suggesting an encouraging antitumor signal in patients.48,49 In combination with immunotherapy, the synergistic effect of ladiratuzumab vedotin with pembrolizumab is being investigated in a single-arm, open-label, phase 1b/2 study.50 The ongoing study including 26 patients with mTNBC showed an ORR of 54% with at least 3 months of follow-up.
In preclinical studies, the bystander killing effect mediated by anti-HER2 ADCs showed the antitumor effect in HER2-low–expressing tumors, including HER2 IHC 1+ or 2+, and HER3-positive tumors.51 Trastuzumab deruxtecan (DS-8201; Enhertu) is a humanized anti-HER2 mAb conjugated with a potent topoisomerase inhibitor, DX-895152; this drug demonstrated antitumor activity in HER2-low–expressing breast cancer, with an ORR of 37.0% and duration of response of 10.4 months in a phase 1b trial.53 Trastuzumab deruxtecan was approved by the FDA for patients with HER2-positive metastatic breast cancer in December 2019, and its efficacy in HER2-low tumors is being assessed in an ongoing phase 3 clinical trial.54 Similarly, ongoing studies are evaluating combination of ADCs with targeted therapies, particularly therapeutic agents synergistic with the payload moiety.55 The summary of various clinical trials and results by biomarkers is outlined in Table 2.
TABLE 2. Summary of Clinical Trials for Metastatic Triple-Negative Breast Cancer and the Efficacy Analysis by Biomarkers
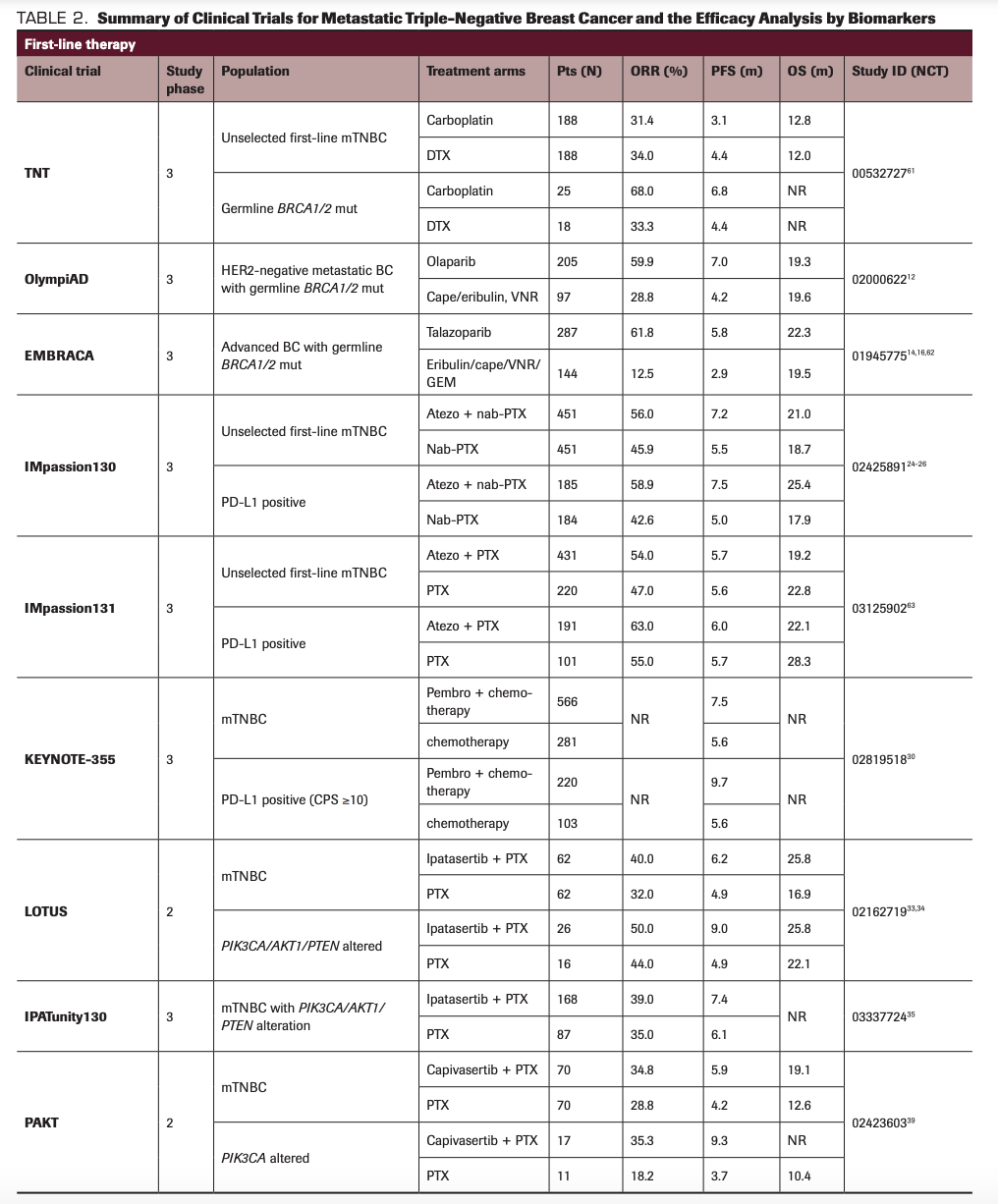
TABLE 2 CONTINUED. Summary of Clinical Trials for Metastatic Triple-Negative Breast Cancer and the Efficacy Analysis by Biomarkers
Other Targets
A number of ongoing clinical trials are targeting key signaling pathways and molecular alterations in TNBC. For example, a subset of TNBC expresses the androgen receptor (AR), and there has been interest in targeting the receptor, similar to prostate cancer.56,57 In a pivotal phase 2 clinical trial, patients with AR-positive TNBC received bicalutamide. However, despite the biomarker selection, the efficacy was modest, with a 6-month CBR of 19%.56 More encouraging results were seen with next-generation potent AR inhibitors such as enzalutamide, with a CBR (16 weeks) of 33% in patients with evaluable AR-positive TNBC.57 Further research is needed to develop better predictive biomarkers as well as combinatorial therapies for AR-positive TNBC. Another approach is to improve tolerability of chemotherapy by developing oral agents such as oral taxanes, as well as drugs like trilaciclib (Cosela) which can put bone marrow cells in G1 cell-cycle arrest, thereby reducing the myelosuppressive toxicity from chemotherapy and improving the efficacy/toxicity ratio.58,59
In summary, over the past few years there have been major strides in both understanding TNBC biology and in targeting TNBC with precision therapies. However, major questions and challenges remain. In particular, in the era of precision medicine, the need exists to develop biomarkers beyond routine genotyping. For example, can we identify patients with HRD-high subtype TNBC who could derive benefit from PARP inhibitors? Can we identify biomarkers to predict the response from single-agent immunotherapy? How can we identify activation of compensatory pathways and overcome resistance to targeted therapies? Successful clinical application of different therapies targeting different subsets of TNBC could potentially transform the therapeutic landscape of mTNBC and make the current definition of TNBC obsolete.
Financial Disclosure: The authors received no financial support for the research, authorship, and/or publication of this article.
AN owns stock of Chugai, Inc. Nagayama’s immediate family member has a leadership position with Chugai, Inc, and Roche, Inc.
NV reports research funding to their institution (Massachusetts General Hospital) from Merck, Daehwa, Novartis, Pfizer (also travel reimbursement for conference presentation of ongoing study), and Radius, as well as prior advisory board participation with AbbVie.
AB reports consultant/advisory board to Genentech/Roche, Immunomedics, Novartis, Pfizer, Merck, Radius Health, Daiichi Sankyo/AstraZeneca, Sanofi, Puma Biotechnology, Phillips; and research funding to the institution: Genentech/Roche, Immunomedics, Novartis, Pfizer, Merck, Radius Health, Sanofi, Mersana.
References
1. Dent R, Trudeau M, Pritchard KI, et al. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res. 2007;13(15 Pt 1):4429-4434. doi:10.1158/1078-0432.CCR-06-3045
2. Lehmann BD, Bauer JA, Chen X, et al. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Invest. 2011;121(7):2750-2767. doi:10.1172/JCI45014
3. Bareche Y, Venet D, Ignatiadis M, et al. Unravelling triple-negative breast cancer molecular heterogeneity using an integrative multiomic analysis. Ann Oncol. 2018;29(4):895-902. doi:10.1093/annonc/mdy024
4. Lehmann BD, Jovanović B, Chen X, et al. Refinement of triple-negative breast cancer molecular subtypes: implications for neoadjuvant chemotherapy selection. PloS One. 2016;11(6):e0157368. doi:10.1371/journal.pone.0157368
5. Staaf J, Glodzik D, Bosch A, et al. Whole-genome sequencing of triple-negative breast cancers in a population-based clinical study. Nat Med. 2019;25(10):1526-1533. doi:10.1038/s41591-019-0582-4
6. Lawrence RT, Perez EM, Hernández D, et al. The proteomic landscape of triple-negative breast cancer. Cell Rep. 2015;11(4):630-644. doi:10.1016/j.celrep.2015.03.050
7. Dieci MV, Barbieri E, Piacentini F, F et al. Discordance in receptor status between primary and recurrent breast cancer has a prognostic impact: a single-institution analysis. Ann Oncol. 2013;24(1):101-108. doi:10.1093/annonc/mds248
8. Angus L, Smid M, Wilting SM, et al. The genomic landscape of metastatic breast cancer highlights changes in mutation and signature frequencies. Nat Genet. 2019;51(10):1450-1458. doi:10.1038/s41588-019-0507-7
9. Karaayvaz M, Cristea S, Gillespie SM, et al. Unravelling subclonal heterogeneity and aggressive disease states in TNBC through single-cell RNA-seq. Nat Commun. 2018;9(1):3588. doi:10.1038/s41467-018-06052-0
10. Pilié PG, Tang C, Mills GB, Yap TA. State-of-the-art strategies for targeting the DNA damage response in cancer. Nature Rev Clin Oncol. 2019;16(2):81-104. doi:10.1038/s41571-018-0114-z
11. Helleday T. The underlying mechanism for the PARP and BRCA synthetic lethality: clearing up the misunderstandings. Mol Oncol. 2011;5(4):387-393. doi:10.1016/j.molonc.2011.07.001
12. Robson M, Im S-A, Senkus E, et al. Olaparib for metastatic breast cancer in patients with a germline BRCA mutation. N Engl J Med. 2017;377(6):523-533. doi:10.1056/NEJMoa1706450
13. Robson ME, Tung N, Conte P, et al. OlympiAD final overall survival and tolerability results: olaparib versus chemotherapy treatment of physician's choice in patients with a germline BRCA mutation and HER2-negative metastatic breast cancer. Ann Oncol. 2019;30(4):558-566. doi:10.1093/annonc/mdz012
14. Litton JK, Rugo HS, Ettl J, et al. Talazoparib in patients with advanced breast cancer and a germline BRCA mutation. N Engl J Med. 2018;379(8):753-763. doi:10.1056/NEJMoa1802905
15. Hurvitz SA, Goncalves A, Rugo HS, et al. Talazoparib in patients with a germline BRCA-mutated advanced breast cancer: detailed safety analyses from the phase III EMBRACA trial. Oncologist. 2020;25(3):e439-e450. doi:10.1634/theoncologist.2019-0493
16. Litton JK, Hurvitz SA, Mina LA, et al. Talazoparib (TALA) in germline BRCA1/2 (gBRCA1/2)-mutated human epidermal growth factor receptor 2 negative (HER2-) advanced breast cancer (ABC): final overall survival (OS) results from randomized phase 3 EMBRACA trial. Cancer Res. 2020;80(16 Supplement):abstr CT071. doi:10.1158/1538-7445.am2020-ct071
17. Rugo HS, Olopade OI, DeMichele A, et al; I-SPY 2 Investigators. Adaptive randomization of veliparib-carboplatin treatment in breast cancer. N Engl J Med. 2016;375(1):23-34. doi:10.1056/NEJMoa1513749
18. Tutt A, Kaufman B, Garber J, et al. OlympiA: a randomized phase III trial of olaparib as adjuvant therapy in patients with high-risk HER2-negative breast cancer (BC) and a germline BRCA1/2 mutation (gBRCAm). Ann Oncol. 2017;28(Suppl 5):v67. doi:10.1093/annonc/mdx362.065
19. Aogi K, Yonemori K, Takahashi M, et al. Efficacy and safety of olaparib combined with eribulin in patients with advanced or metastatic triple negative breast cancer (TNBC) previously treated with anthracyclines and taxanes: the final analysis of a Japanese phase I/II trial. Ann Oncol. 2017;28(Suppl 5):v81. doi:10.1093/annonc/mdx365.014
20. Chopra N, Tovey H, Pearson A, et al. Homologous recombination DNA repair deficiency and PARP inhibition activity in primary triple negative breast cancer. Nat Commun. 2020;11(1):2662. doi:10.1038/s41467-020-16142-7
21. Tung NM, Robson ME, Ventz S, et al; Translational Breast Cancer Research Consortium. TBCRC 048: a phase II study of olaparib monotherapy in metastatic breast cancer patients with germline or somatic mutations in DNA damage response (DDR) pathway genes (Olaparib Expanded). J Clin Oncol. 2020;38(15 suppl):abstr 1002. doi:10.1200/JCO.2020.38.15_suppl.1002
22. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252-264. doi:10.1038/nrc3239
23. Emens LA, Cruz C, Eder JP, et al. Long-term clinical outcomes and biomarker analyses of atezolizumab therapy for patients with metastatic triple-negative breast cancer: a phase 1 study. JAMA Oncol. 2019;5(1):74-82. doi:10.1001/jamaoncol.2018.4224
24. Schmid P, Adams S, Rugo HS, et al; IMpassion130 Trial Investigators. Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N Engl J Med. 2018;379(22):2108-2121. doi:10.1056/NEJMoa1809615
25. Schmid P, Adams S, Rugo HS, et al. IMpassion130: updated overall survival (OS) from a global, randomized, double-blind, placebo-controlled, phase III study of atezolizumab (atezo) + nab-paclitaxel (nP) in previously untreated locally advanced or metastatic triple-negative breast cancer (mTNBC). J Clin Oncol. 2019;37(15 suppl):abstr 1003. doi:10.1200/JCO.2019.37.15_suppl.1003
26. Emens LA, Adams S, Barrios CH, et al. LBA16 IMpassion130: final OS analysis from the pivotal phase III study of atezolizumab + nab-paclitaxel vs placebo + nab-paclitaxel in previously untreated locally advanced or metastatic triple-negative breast cancer. Ann Oncol. 2020;31(Suppl 4):S1148. doi:10.1016/j.annonc.2020.08.2244
27. Rugo HS, Loi S, Adams S, et al. LBA20 - performance of PD-L1 immunohistochemistry (IHC) assays in unresectable locally advanced or metastatic triple-negative breast cancer (mTNBC): post-hoc analysis of IMpassion130. Ann Oncol. 2019;30(Suppl 5):v858-v859. doi:10.1093/annonc/mdz394.009
28. Davis AA, Patel VG. The role of PD-L1 expression as a predictive biomarker: an analysis of all US Food and Drug Administration (FDA) approvals of immune checkpoint inhibitors. J Immunother Cancer. 2019;7(1):278. doi:10.1186/s40425-019-0768-9
29. Cortes Castan J, Guo Z, Karantza V, Aktan G. KEYNOTE-355: randomized, double-blind, phase III study of pembrolizumab (pembro) + chemotherapy (chemo) vs placebo (pbo) + chemo for previously untreated, locally recurrent, inoperable or metastatic triple-negative breast cancer (mTNBC). Ann Oncol. 2017;28(Suppl 10):x25. doi:10.1093/annonc/mdx656
30. Cortes J, Cescon DW, Rugo HS, et al. KEYNOTE-355: randomized, double-blind, phase III study of pembrolizumab + chemotherapy versus placebo + chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer. J Clin Oncol. 2020;38(15 Suppl):abstr 1000. doi:10.1200/JCO.2020.38.15_suppl.1000
31. Perou CM, Sørlie T, Eisen MB, et al. Molecular portraits of human breast tumours. Nature. 2000;406(6797):747-752. doi:10.1038/35021093
32. Curtis C, Shah SP, Chin SF, et al. The genomic and transcriptomic architecture of 2,000 breast tumours reveals novel subgroups. Nature. 2012;486(7403):346-352. doi:10.1038/nature10983
33. Kim S-B, Dent R, Im S-A, et al; LOTUS Investigators. Ipatasertib plus paclitaxel versus placebo plus paclitaxel as first-line therapy for metastatic triple-negative breast cancer (LOTUS): a multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol. 2017;18(10):1360-1372. doi:10.1016/S1470-2045(17)30450-3
34. Dent R, Oliveira M, Isakoff SJ, et al. 139O Final results of the double-blind placebo (PBO)-controlled randomised phase II LOTUS trial of first-line ipatasertib (IPAT) + paclitaxel (PAC) for inoperable locally advanced/metastatic triple-negative breast cancer (mTNBC). Ann Oncol. 2020;31(suppl 2):S64-SS5. doi:10.1016/j.annonc.2020.03.240
35. Dent R, Kim S-B, Oliveira M, et al. Double-blind placebo (PBO)-controlled randomized phase III trial evaluating first-line ipatasertib (IPAT) combined with paclitaxel (PAC) for PIK3CA/AKT1/PTEN-altered locally advanced unresectable or metastatic triple-negative breast cancer (aTNBC): primary results from IPATunity130 Cohort A. Cancer Res. 2021;81(4 Suppl):abstr GS3-04. doi:10.1158/1538-7445.SABCS20-GS3-04
36. Martin M, Chan A, Dirix L, et al. A randomized adaptive phase II/III study of buparlisib, a pan-class I PI3K inhibitor, combined with paclitaxel for the treatment of HER2- advanced breast cancer (BELLE-4). Ann Oncol. 2017;28(2):313-320. doi:10.1093/annonc/mdw562
37. Davies BR, Greenwood H, Dudley P, et al. Preclinical pharmacology of AZD5363, an inhibitor of AKT: pharmacodynamics, antitumor activity, and correlation of monotherapy activity with genetic background. Mol Cancer Ther. 2012;11(4):873-887. doi:10.1158/1535-7163.MCT-11-0824-T
38. Addie M, Ballard P, Buttar D, et al. Discovery of 4-amino-N-[(1S)-1-(4-chlorophenyl)-3-hydroxypropyl]-1-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)piperidine-4-carboxamide (AZD5363), an orally bioavailable, potent inhibitor of Akt kinases. J Med Chem. 2013;56(5):2059-2073. doi:10.1021/jm301762v
39. Schmid P, Abraham J, Chan S, et al. Capivasertib plus paclitaxel versus placebo plus paclitaxel as first-line therapy for metastatic triple-negative breast cancer: the PAKT trial. J Clin Oncol. 2020;38(5):423-433. doi:10.1200/JCO.19.00368
40. Schmid P, Cortes J, Robson M, et al. Capivasertib and paclitaxel in first-line treatment of patients with metastatic triple-negative breast cancer: a phase III trial (CAPItello-290). Cancer Res. 2020;80(4 Suppl):abstr OT2-08-2. doi:10.1158/1538-7445.sabcs19-ot2-08-02
41. Nagayama A, Ellisen LW, Chabner B, Bardia A. Antibody-drug conjugates for the treatment of solid tumors: clinical experience and latest developments. Target Oncol. 2017;12(6):719-739. doi:10.1007/s11523-017-0535-0
42. Junttila TT, Li G, Parsons K, Lewis Phillips G, Sliwkowski MX. Trastuzumab-DM1 (T-DM1) retains all the mechanisms of action of trastuzumab and efficiently inhibits growth of lapatinib insensitive breast cancer. Breast Cancer Res Treat. 2011;128(2):347-356. doi:10.1007/s10549-010-1090-x
43. Staudacher AH, Brown MP. Antibody drug conjugates and bystander killing: is antigen-dependent internalisation required? Br J Cancer. 2017;117(12):1736-1742. doi:10.1038/bjc.2017.367
44. Goldenberg DM, Cardillo TM, Govindan SV, Rossi EA, Sharkey RM. Trop-2 is a novel target for solid cancer therapy with sacituzumab govitecan (IMMU-132), an antibody-drug conjugate (ADC). Oncotarget. 2015;6(26):22496-22512. doi:10.18632/oncotarget.4318
45. Bardia A, Mayer IA, Vahdat LT, Tolaney SM, Isakoff SJ, Diamond JR et al. Sacituzumab govitecan-hziy in refractory metastatic triple-negative breast cancer. N Engl J Med. 2019;380(8):741-751. doi:10.1056/NEJMoa1814213
46. Bardia A, Tolaney SM, Loirat D, et al. LBA17 ASCENT: a randomized phase III study of sacituzumab govitecan (SG) vs treatment of physician's choice (TPC) in patients (pts) with previously treated metastatic triple-negative breast cancer (mTNBC). Ann Oncol. 2020;31(Suppl 4):S1149-S1150. doi:10.1016/j.annonc.2020.08.2245
47. Sussman D, Smith LM, Anderson ME, et al. SGN-LIV1A: a novel antibody-drug conjugate targeting LIV-1 for the treatment of metastatic breast cancer. Mol Cancer Ther. 2014;13(12):2991-3000. doi:10.1158/1535-7163.MCT-13-0896
48. Forero A, Burris H III, Mita M, et al. Interim analysis of a phase 1 study of the antibody-drug conjugate SGN-LIV1A in patients with metastatic breast cancer. Cancer Res. 2016;76(Suppl 4):abstr P3-14-05. doi:10.1158/1538-7445.sabcs15-p3-14-05
49. Modi S, Pusztai L, Forero A, et al. Phase 1 study of the antibody-drug conjugate SGN-LIV1A in patients with heavily pretreated triple-negative metastatic breast cancer. Cancer Res. 2018;78(Suppl 4):abstr PD3-14. doi:10.1158/1538-7445.sabcs17-pd3-14
50. Han HS, Alemany CA, Brown-Glaberman UA, et al. SGNLVA-002: single-arm, open label phase Ib/II study of ladiratuzumab vedotin (LV) in combination with pembrolizumab for first-line treatment of patients with unresectable locally advanced or metastatic triple-negative breast cancer. J Clin Oncol. 2019;37(15 Suppl):abstr TPS1110. doi:10.1200/JCO.2019.37.15_suppl.TPS1110
51. Ogitani Y, Hagihara K, Oitate M, Naito H, Agatsuma T. Bystander killing effect of DS-8201a, a novel anti-human epidermal growth factor receptor 2 antibody-drug conjugate, in tumors with human epidermal growth factor receptor 2 heterogeneity. Cancer Sci. 2016;107(7):1039-1046. doi:10.1111/cas.12966
52. Ogitani Y, Aida T, Hagihara K, et al. DS-8201a, a novel HER2-targeting ADC with a novel DNA topoisomerase I inhibitor, demonstrates a promising antitumor efficacy with differentiation from T-DM1. Clin Cancer Res. 2016;22(20):5097-5108. doi:10.1158/1078-0432.CCR-15-2822
53. Modi S, Park H, Murthy RK, et al. Antitumor activity and safety of trastuzumab deruxtecan in patients with HER2-low-expressing advanced breast cancer: results from a phase Ib study. J Clin Oncol. 2020;38(17):1887-1896. doi:10.1200/JCO.19.02318
54. Modi S, Ohtani S, Lee CC, Wang K, Saxena K, Cameron DA. A phase III, multicenter, randomized, open label trial of [fam-] trastuzumab deruxtecan (DS-8201a) versus investigator’s choice in HER2-low breast cancer. J Clin Oncol. 2019;37(15 Suppl):abstr TPS1102. doi:10.1200/JCO.2019.37.15_suppl.TPS1102
55. Bardia A, Spring LM, Juric D, et al. 358TiP phase Ib/II study of antibody-drug conjugate, sacituzumab govitecan, in combination with the PARP inhibitor, talazoparib, in metastatic triple-negative breast cancer. Ann Oncol. 2020;31(suppl 4):S394. doi:10.1016/j.annonc.2020.08.460
56. Gucalp A, Tolaney S, Isakoff SJ, et al; Translational Breast Cancer Research Consortium (TBCRC 011). Phase II trial of bicalutamide in patients with androgen receptor-positive, estrogen receptor-negative metastatic breast cancer. Clin Cancer Res. 2013;19(19):5505-5512. doi:10.1158/1078-0432.CCR-12-3327
57. Traina TA, Miller K, Yardley DA, et al. Enzalutamide for the treatment of androgen receptor-expressing triple-negative breast cancer. J Clin Oncol. 2018;36(9):884-890. doi:10.1200/JCO.2016.71.3495
58. Kang Y-K, Ryu M-H, Park SH, et al. Efficacy and safety findings from DREAM: a phase III study of DHP107 (oral paclitaxel) versus i.v. paclitaxel in patients with advanced gastric cancer after failure of first-line chemotherapy. Ann Oncol. 2018;29(5):1220-1226. doi:10.1093/annonc/mdy055
59. Tan AR, Wright GS, Thummala AR, et al. Trilaciclib plus chemotherapy versus chemotherapy alone in patients with metastatic triple-negative breast cancer: a multicentre, randomised, open-label, phase 2 trial. Lancet Oncol. 2019;20(11):1587-1601. doi:10.1016/S1470-2045(19)30616-3
60. Burstein MD, Tsimelzon A, Poage GM, et al. Comprehensive genomic analysis identifies novel subtypes and targets of triple-negative breast cancer. Clin Cancer Res. 2015;21(7):1688-1698. doi:10.1158/1078-0432.CCR-14-0432
61. Tutt A, Tovey H, Cheang MCU, et al. Carboplatin in BRCA1/2-mutated and triple-negative breast cancer BRCAness subgroups: the TNT Trial. Nat Med. 2018;24(5):628-637. doi:10.1038/s41591-018-0009-7
62. Rugo HS, Ettl J, Hurvitz SA, et al. Outcomes in clinically relevant patient subgroups from the EMBRACA study: talazoparib vs physician's choice standard-of-care chemotherapy. JNCI Cancer Spectr. 2020;4(1):pkz085. doi:10.1093/jncics/pkz085
63. Miles DW, Gligorov J, André F, et al. LBA15 Primary results from IMpassion131, a double-blind placebo-controlled randomised phase III trial of first-line paclitaxel (PAC) ± atezolizumab (atezo) for unresectable locally advanced/metastatic triple-negative breast cancer (mTNBC). Ann Oncol. 2020;31(suppl 4):S1147-S1148. doi:10.1016/j.annonc.2020.08.2243
64. Cortes J, Lipatov O, Im SA, et al. KEYNOTE-119: phase III study of pembrolizumab (pembro) versus single-agent chemotherapy (chemo) for metastatic triple negative breast cancer (mTNBC). Ann Oncol. 2019;30(suppl 5):v859-v860. doi:10.1093/annonc/mdz.394
