Brain Metastasis from Unknown Primary Tumour: Moving from Old Retrospective Studies to Clinical Trials on Targeted Agents
Abstract
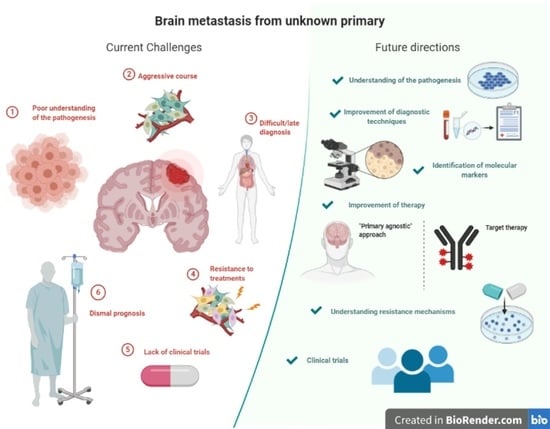
:Simple Summary
Abstract
1. Introduction
2. Methods
3. Definition and Diagnosis of CUP
4. Pathogenesis of CUP
5. The Role of Immunohistochemistry
6. Gene Expression Profiling: A New Frontier
7. Management
7.1. Outcome
7.2. Standard Treatments
7.3. Innovative Treatments: Case Reports
| Ref | Age | Presenting Symptoms | Location | Histopathology | Other Findings | Treatment | Primary Found during Follow-Up | Survival |
|---|---|---|---|---|---|---|---|---|
| Yamasaki et al., 2018 [71] | 67 | Right lower extremity paresis, dysarthria, memory loss. | Bilateral multiple cerebral lesions | Adenocarcinoma CK7 and TTF-1 +, | 147 pack-year smoking history. CEA level029.6ng/mL | Oral erlotinib (150 mg/day). | No | >8 months. Significant improvement of brain and other metastases |
| Huang et al., 2018 [65] | 35 | Headache and lower back pain | leptomeningeal metastases + spinal cord | Adenocarcinoma CK7, CDX2, and PAX-8 + TTF-1, CK20, ER/PR – No ALK rearrangements, EGFR and BRAF mutations, microsatellite stable. FISH demonstrating HER2 positivity on dual probe (HER2/CEP17 ratio 5.08). | CSF cytology = ctDNA analysis found amplifications of HER2 and MPL, mutations in the PIK3CA, CDKN2A and TP53 genes | Ado-trastuzumab emtansine + intrathecal trastuzumab and oral lapatinib | No | Clinical improvement, reduction of tumour markers and negative follow-up CSF cytology. >2 years |
| Proboka et al., 2018 [72] | 60 | Dizziness after movements and increased fatigue | Brainstem | Metastatic melanoma. weakly positive for HMB-45 and Melan A, strongly positive for MART-1, S-100, and vimentin, and that the Ki-67 index was 35%. BRAF gene mutations in codons V600E, V600K, and V600D were not detected. | NS | Surgery + ECHO-7 Oncolytic Virus Rigvir | No | Follow-up imaging was stable > 3 years |
| Mahase et al., 2017 [73] | 84 | Sudden-onset dysgraphia, right limbs weakness, confusion | Multiple bilateral lesions | Adenocarcinoma CK7, TTF-1 ER + CK20, PR, HER2-neu, CDX-2, WT-1, EGFR - | NS | SRS+ Craniotomy + implantation of Cs-131 in the surgical cavity | No | Alive at 6 years follow-up |
| Kuwata et al., 2011 [74] | 69 | Dizziness | Cerebellar + multiple smaller brain tumours | Adenocarcinoma, Napsin A and TTF-1 + EGFR exon 19 deletion | Serum CEA= 129.9 ng/mL; SLX = 150 U/mL. Other tumour markers normal range. | Surgical resection of cerebellar lesion + GKRS on other. Gefitinib (250 mg/day). | No | At 46 days follow-up shrinkage of LN and reduction of markers. Survival NS |
7.4. Innovative Treatments: Ongoing Clinical Trials
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Achrol, A.S.; Rennert, R.C.; Anders, C.; Soffietti, R.; Ahluwalia, M.S.; Nayak, L.; Peters, S.; Arvold, N.D.; Harsh, G.R.; Steeg, P.S.; et al. Brain metastases. Nat. Rev. Dis. Primer 2019, 5, 5. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network(NCCN). NCCN Clinical Practice Guidelines in Oncology Occult Primary Version 2; NCCN: Houston, TX, USA, 2020. [Google Scholar]
- Pavlidis, N.; Pentheroudakis, G. Cancer of unknown primary site. Lancet Engl. 2012, 379, 1428–1435. [Google Scholar] [CrossRef]
- Chee, C.P.; Byrnes, D.P. Cases of brain metastasis presenting as the first sign of systemic cancer. Singap. Med. J. 1988, 29, 252–256. [Google Scholar]
- Giordana, M.T.; Cordera, S.; Boghi, A. cerebral metastases as first symptom of cancer: A clinico-pathologic study. J. Neurooncol. 2000, 50, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Mavrakis, A.N.; Halpern, E.F.; Barker, F.G.; Gonzalez, R.G.; Henson, J.W. Diagnostic evaluation of patients with a brain mass as the presenting manifestation of cancer. Neurology 2005, 65, 908–911. [Google Scholar] [CrossRef]
- Merchut, M.P. Brain metastases from undiagnosed systemic neoplasms. Arch. Intern. Med. 1989, 149, 1076–1080. [Google Scholar] [CrossRef]
- Niranjan, A.; Kano, H.; Khan, A.; Kim, I.-Y.; Kondziolka, D.; Flickinger, J.C.; Lunsford, L.D. Radiosurgery for brain metastases from unknown primary cancers. Int. J. Radiat. Oncol. Biol. Phys. 2010, 77, 1457–1462. [Google Scholar] [CrossRef]
- van de Pol, M.; van Aalst, V.C.; Wilmink, J.T.; Twijnstra, A. Brain metastases from an unknown primary tumour: Which diagnostic procedures are indicated? J. Neurol. Neurosurg. Psychiatry 1996, 61, 321–323. [Google Scholar] [CrossRef] [Green Version]
- Salvati, M.; Cervoni, L.; Raco, A. Single brain metastases from unknown primary malignancies in CT-era. J. Neurooncol. 1995, 23, 75–80. [Google Scholar] [CrossRef]
- Wolpert, F.; Weller, M.; Berghoff, A.S.; Rushing, E.; Füreder, L.M.; Petyt, G.; Leske, H.; Andratschke, N.; Regli, L.; Neidert, M.C.; et al. Diagnostic value of 18F-fluordesoxyglucose positron emission tomography for patients with brain metastasis from unknown primary site. Eur. J. Cancer Oxf. Engl. 1990 2018, 96, 64–72. [Google Scholar] [CrossRef]
- Thomas, A.J.; Rock, J.P.; Johnson, C.C.; Weiss, L.; Jacobsen, G.; Rosenblum, M.L. Survival of patients with synchronous brain metastases: An epidemiological study in southeastern Michigan. J. Neurosurg. 2000, 93, 927–931. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dhopesh, V.P.; Yagnik, P.M. Brain metastasis: Analysis of patients without known cancer. South. Med. J. 1985, 78, 171–172. [Google Scholar] [CrossRef] [PubMed]
- Bartelt, S.; Lutterbach, J. Brain Metastases in Patients with Cancer of Unknown Primary. J. Neuro Oncol. 2003, 3, 249–253. [Google Scholar] [CrossRef] [PubMed]
- Chee, C.P. Brain metastasis of unknown origin. Singap. Med. J. 1990, 31, 48–50. [Google Scholar]
- Lagerwaard, F.; Levendag, P.; Nowak, P.C.; Eijkenboom, W.H.; Hanssens, P.J.; Schmitz, P.M. Identification of prognostic factors in patients with brain metastases: A review of 1292 patients. Int. J. Radiat. Oncol. 1999, 43, 795–803. [Google Scholar] [CrossRef]
- Maesawa, S.; Kondziolka, D.; Thompson, T.P.; Flickinger, J.C.; Dade, L. Brain metastases in patients with no known primary tumor. Cancer 2000, 89, 1095–1101. [Google Scholar] [CrossRef]
- Nguyen, L.N.; Maor, M.H.; Oswald, M.J. Brain metastases as the only manifestation of an undetected primary tumor. Cancer 1998, 83, 2181–2184. [Google Scholar] [CrossRef]
- Agazzi, S.; Pampallona, S.; Pica, A.; Vernet, O.; Regli, L.; Porchet, F.; Villemure, J.G.; Leyvraz, S. The origin of brain metastases in patients with an undiagnosed primary tumour. Acta Neurochir. (Wien) 2004, 146, 153–157. [Google Scholar] [CrossRef]
- Akimoto, J.; Fukuhara, H.; Suda, T.; Nagai, K.; Ichikawa, M.; Fukami, S.; Kohno, M.; Matsubayashi, J.; Nagao, T. Clinicopathological analysis in patients with neuroendocrine tumors that metastasized to the brain. BMC Cancer 2016, 16, 36. [Google Scholar] [CrossRef] [Green Version]
- Chevalier, T.L.; Smith, F.P.; Caille, P.; Constans, J.P.; Rouesse, J.G. Sites of primary malignancies in patients presenting with cerebral metastases. A review of 120 cases. Cancer 1985, 56, 880–882. [Google Scholar] [CrossRef]
- D’Ambrosio, A.L.; Agazzi, S. Prognosis in patients presenting with brain metastasis from an undiagnosed primary tumor. Neurosurg. Focus 2007, 22, E7. [Google Scholar] [CrossRef] [PubMed]
- Eapen, L.; Vachet, M.; Catton, G.; Danjoux, C.; McDermot, R.; Nair, B.; Girard, A.; Genest, P.; Stewart, D.; Gerig, L. Brain metastases with an unknown primary: A clinical perspective. J. Neurooncol. 1988, 6, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Füreder, L.M.; Widhalm, G.; Gatterbauer, B.; Dieckmann, K.; Hainfellner, J.A.; Bartsch, R.; Zielinski, C.C.; Preusser, M.; Berghoff, A.S. Brain metastases as first manifestation of advanced cancer: Exploratory analysis of 459 patients at a tertiary care center. Clin. Exp. Metastasis 2018, 35, 727–738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, H.J.; Chang, W.S.; Jung, H.H.; Park, Y.G.; Kim, H.Y.; Chang, J.H. Optimal treatment decision for brain metastases of unknown primary origin: The role and timing of radiosurgery. Brain Tumor Res. Treat. 2016, 4, 107–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koç, Z.P.; Kara, P.Ö.; Dağtekin, A. Detection of unknown primary tumor in patients presented with brain metastasis by F-18 fluorodeoxyglucose positron emission tomography/computed tomography. CNS Oncol. 2018, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsunaga, S.; Shuto, T.; Kobayashi, N. Gamma Knife Radiosurgery for Metastatic Brain Tumors from Cancer of Unknown Primary. World Neurosurg. 2019, 122, e1465–e1471. [Google Scholar] [CrossRef]
- Zimm, S.; Wampler, G.L.; Stablein, D.; Hazra, T.; Young, H.F. Intracerebral metastases in solid-tumor patients: Natural history and results of treatment. Cancer 1981, 48, 384–394. [Google Scholar] [CrossRef]
- Khansur, T.; Routh, A.; Hickman, B. Brain metastases from unknown primary site. J. Miss. State Med. Assoc. 1997, 38, 238–242. [Google Scholar]
- Roh, T.H.; Choi, M.S.; You, N.; Jeong, D.; Jang, A.H.; Seo, M.R.; Lee, S.R.; Kim, S.-H. Identifying candidates for gamma knife radiosurgery among elderly patients with brain metastases. J. Neurooncol. 2018, 137, 559–565. [Google Scholar] [CrossRef]
- Dziggel, L.; Bajrovic, A.; Schild, S.E.; Rades, D. Stereotactic radiosurgery alone for one to two brain metastases from cancer of unknown primary. Anticancer Res. 2018, 38, 565–567. [Google Scholar]
- Rades, D.; Bohlen, G.; Lohynska, R.; Veninga, T.; Stalpers, L.J.A.; Schild, S.E.; Dunst, J. Whole-brain radiotherapy with 20 Gy in 5 Fractions for brain metastases in patients with cancer of unknown primary (CUP). Strahlenther. Onkol. 2007, 183, 631–636. [Google Scholar] [CrossRef] [PubMed]
- Berghoff, A.S.; Schur, S.; Füreder, L.M.; Gatterbauer, B.; Dieckmann, K.; Widhalm, G.; Hainfellner, J.; Zielinski, C.C.; Birner, P.; Bartsch, R.; et al. Descriptive statistical analysis of a real life cohort of 2419 patients with brain metastases of solid cancers. ESMO Open 2016, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meiri, E.; Mueller, W.C.; Rosenwald, S.; Zepeniuk, M.; Klinke, E.; Edmonston, T.B.; Werner, M.; Lass, U.; Barshack, I.; Feinmesser, M.; et al. A second-generation microRNA-based assay for diagnosing tumor tissue origin. Oncologist 2012, 17, 801–812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mueller, W.C.; Spector, Y.; Edmonston, T.B.; St Cyr, B.; Jaeger, D.; Lass, U.; Aharonov, R.; Rosenwald, S.; Chajut, A. Accurate classification of metastatic brain tumors using a novel microRNA-based test. Oncologist 2011, 16, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, M.; Barfod, B.E.; Urakawa, Y. Gamma Knife Radiosurgery for Brain Metastases of Non-Lung Cancer Origin: Focusing on Multiple Brain Lesions. Jpn. Exp. Gamma Knife Radiosurg. 2009, 22, 154–169. [Google Scholar] [CrossRef]
- Kased, N.; Huang, K.; Nakamura, J.L.; Sahgal, A.; Larson, D.A.; McDermott, M.W.; Sneed, P.K. Gamma Knife radiosurgery for brainstem metastases: The UCSF experience. J. Neurooncol. 2008, 86, 195–205. [Google Scholar] [CrossRef]
- Rades, D.; Dziggel, L.; Janssen, S.; Khoa, M.T.; Duong, V.N.; Khiem, V.H.; Gebauer, N.; Bartscht, T.; Schild, S.E. Predictive factors for local control and survival in patients with cancer of unknown primary (CUP) irradiated for cerebral metastases. Anticancer Res. 2018, 38, 2415–2418. [Google Scholar] [CrossRef]
- Drlicek, M.; Bodenteich, A.; Urbanits, S.; Grisold, W. Immunohistochemical panel of antibodies in the diagnosis of brain metastases of the unknown primary. Pathol. Res. Pract. 2004, 200, 727–734. [Google Scholar] [CrossRef]
- Petrovich, Z.; Yu, C.; Giannotta, S.L.; O’Day, S.; Apuzzo, M.L.J. Survival and pattern of failure in brain metastasis treated with stereotactic gamma knife radiosurgery. J. Neurosurg. 2002, 97, 499–506. [Google Scholar] [CrossRef]
- Yuile, P.G.; Tran, M.H. Survival with brain metastases following radiation therapy. Australas. Radiol. 2002, 46, 390–395. [Google Scholar] [CrossRef]
- Hall, W.A.; Djalilian, H.R.; Nussbaum, E.S.; Cho, K.H. Long-term survival with metastatic cancer to the brain. Med. Oncol. 2000, 17, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Nussbaum, E.S.; Djalilian, H.R.; Cho, K.H.; Hall, W.A. Brain metastases: Histology, multiplicity, surgery, and survival. Cancer 1996, 78, 1781–1788. [Google Scholar] [CrossRef]
- Debevec, M. Management of patients with brain metastases of unknown origin. Neoplasma 1990, 37, 601–606. [Google Scholar] [PubMed]
- Yardeni, D.; Reichenthal, E.; Zucker, G.; Rubeinstein, A.; Cohen, M.; Israeli, V.; Shalit, M.N. Neurosurgical management of single brain metastasis. Surg. Neurol. 1984, 21, 377–384. [Google Scholar] [CrossRef]
- Ebels, E.J.; van der Meulen, J.D.M. Cerebral metastasis without known primary tumour: A retrospective study. Clin. Neurol. Neurosurg. 1978, 80, 195–197. [Google Scholar] [CrossRef]
- Urban, D.; Rao, A.; Bressel, M.; Lawrence, Y.R.; Mileshkin, L. Cancer of unknown primary: A population-based analysis of temporal change and socioeconomic disparities. Br. J. Cancer 2013, 109, 1318–1324. [Google Scholar] [CrossRef] [Green Version]
- Binder, C.; Matthes, K.L.; Korol, D.; Rohrmann, S.; Moch, H. Cancer of unknown primary—Epidemiological trends and relevance of comprehensive genomic profiling. Cancer Med. 2018, 7, 4814–4824. [Google Scholar] [CrossRef]
- Al-Brahim, N.; Ross, C.; Carter, B.; Chorneyko, K. The value of postmortem examination in cases of metastasis of unknown origin-20-year retrospective data from a tertiary care center. Ann. Diagn. Pathol. 2005, 9, 77–80. [Google Scholar] [CrossRef]
- Fizazi, K.; Greco, F.A.; Pavlidis, N.; Daugaard, G.; Oien, K.; Pentheroudakis, G. ESMO Guidelines Committee Cancers of unknown primary site: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2015, 26 (Suppl. 5), v133–v138. [Google Scholar] [CrossRef]
- Soffietti, R.; Abacioglu, U.; Baumert, B.; Combs, S.E.; Kinhult, S.; Kros, J.M.; Marosi, C.; Metellus, P.; Radbruch, A.; Villa Freixa, S.S.; et al. Diagnosis and treatment of brain metastases from solid tumors: Guidelines from the European Association of Neuro-Oncology (EANO). Neuro Oncol. 2017, 19, 162–174. [Google Scholar] [CrossRef] [Green Version]
- Thapa, P.; Kalshetty, A.; Basu, S. 18F-fluorodeoxyglucose positron emission tomography/computed tomography in carcinoma of unknown primary: A subgroup-specific analysis based on clinical presentation. World J. Nucl. Med. 2018, 17, 219–222. [Google Scholar] [CrossRef]
- Gupta, N.C.; Nicholson, P.; Bloomfield, S.M. FDG-PET in the Staging Work-Up of Patients with Suspected Intracranial Metastatic Tumors. Ann. Surg. 1999, 230, 202. [Google Scholar] [CrossRef]
- Jeong, H.-J.; Chung, J.-K.; Kim, Y.K.; Kim, C.Y.; Kim, D.G.; Jeong, J.M.; Lee, D.S.; Jung, H.W.; Lee, M.C. Usefulness of whole-body 18F-FDG PET in patients with suspected metastatic brain tumors. J. Nucl. Med. 2002, 43, 1432–1437. [Google Scholar] [PubMed]
- Rassy, E.; Assi, T.; Pavlidis, N. Exploring the biological hallmarks of cancer of unknown primary: Where do we stand today? Br. J. Cancer 2020, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Vikeså, J.; Møller, A.K.H.; Kaczkowski, B.; Borup, R.; Winther, O.; Henao, R.; Krogh, A.; Perell, K.; Jensen, F.; Daugaard, G.; et al. Cancers of unknown primary origin (CUP) are characterized by chromosomal instability (CIN) compared to metastasis of know origin. BMC Cancer 2015, 15, 151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davoli, T.; Uno, H.; Wooten, E.C.; Elledge, S.J. Tumor aneuploidy correlates with markers of immune evasion and with reduced response to immunotherapy. Science 2017, 355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bochtler, T.; Krämer, A. Does cancer of unknown primary (CUP) truly exist as a distinct cancer entity? Front. Oncol. 2019, 9. [Google Scholar] [CrossRef] [Green Version]
- Hemminki, K.; Sundquist, K.; Sundquist, J.; Hemminki, A.; Ji, J. Location of metastases in cancer of unknown primary are not random and signal familial clustering. Sci. Rep. 2016, 6, 22891. [Google Scholar] [CrossRef] [Green Version]
- Klee, B.; Law, I.; Højgaard, L.; Kosteljanetz, M. Detection of unknown primary tumours in patients with cerebral metastases using whole-body 18F-flouorodeoxyglucose positron emission tomography. Eur. J. Neurol. 2002, 9, 657–662. [Google Scholar] [CrossRef]
- Rudà, R.; Borgognone, M.; Benech, F.; Vasario, E.; Soffietti, R. Brain metastases from unknown primary tumour. J. Neurol. 2001, 248, 394–398. [Google Scholar] [CrossRef]
- Weiss, L.M.; Chu, P.; Schroeder, B.E.; Singh, V.; Zhang, Y.; Erlander, M.G.; Schnabel, C.A. Blinded comparator study of immunohistochemical analysis versus a 92-gene cancer classifier in the diagnosis of the primary site in metastatic tumors. J. Mol. Diagn. 2013, 15, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Liu, H.; Chen, J.; Chen, H.; Xia, J.; Wang, O.; Xie, J.; Li, M.; Guo, Z.; Chen, G. Identification of the origin of brain metastases based on the relative methylation orderings of CpG sites. Epigenetics 2020. [Google Scholar] [CrossRef]
- Boire, A.; Brandsma, D.; Brastianos, P.K.; Le Rhun, E.; Ahluwalia, M.; Junck, L.; Glantz, M.; Groves, M.D.; Lee, E.Q.; Lin, N.; et al. Liquid biopsy in central nervous system metastases: A RANO review and proposals for clinical applications. Neuro Oncol. 2019, 21, 571–584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, W.-T.; Lu, N.-M.; Hsu, W.-Y.; Chang, S.-E.; Atkins, A.; Mei, R.; Javey, M. CSF-ctDNA SMSEQ Analysis to Tailor the Treatment of a Patient with Brain Metastases: A Case Report. Case Rep. Oncol. 2018, 11, 68–74. [Google Scholar] [CrossRef]
- Gough, M.; Nielsen, M.; Coulter, I.C.; Holliman, D. Survival outcomes following craniotomy for intracranial metastases from an unknown primary. Int. J. Clin. Oncol. 2020, 25, 1475–1482. [Google Scholar] [CrossRef]
- Riihimäki, M.; Thomsen, H.; Hemminki, A.; Sundquist, K.; Hemminki, K. Comparison of survival of patients with metastases from known versus unknown primaries: Survival in metastatic cancer. BMC Cancer 2013, 13, 36. [Google Scholar] [CrossRef] [Green Version]
- Rassy, E.; Zanaty, M.; Azoury, F.; Pavlidis, N. Advances in the management of brain metastases from cancer of unknown primary. Future Oncol. 2019, 15, 2759–2768. [Google Scholar] [CrossRef]
- Wang, L.G.; Guo, Y.; Zhang, X.; Song, S.J.; Xia, J.L.; Fan, F.Y.; Shi, M.; Wei, L.C. Brain metastasis: Experience of the Xi-Jing hospital. Stereotact. Funct. Neurosurg. 2002, 78, 70–83. [Google Scholar] [CrossRef]
- Rades, D.; Nguyen, T.; Khoa, M.T.; Janssen, S.; Schild, S.E. Remaining lifespan of patients aged ≥65 years receiving whole-brain irradiation for metastases from cancer of unknown primary. Anticancer Res. 2020, 40, 2261–2264. [Google Scholar] [CrossRef]
- Yamasaki, M.; Funaishi, K.; Saito, N.; Sakano, A.; Fujihara, M.; Daido, W.; Ishiyama, S.; Deguchi, N.; Taniwaki, M.; Ohashi, N.; et al. Putative lung adenocarcinoma with epidermal growth factor receptor mutation presenting as carcinoma of unknown primary site: A case report. Medicine (Baltimore) 2018, 97, e9942. [Google Scholar] [CrossRef]
- Proboka, G.; Tilgase, A.; Isajevs, S.; Rasa, A.; Alberts, P. Melanoma Unknown Primary Brain Metastasis Treatment with ECHO-7 Oncolytic Virus Rigvir: A Case Report. Front. Oncol. 2018, 8, 43. [Google Scholar] [CrossRef] [PubMed]
- Mahase, S.S.; Julie, D.; Pannullo, S.C.; Parashar, B.; Wernicke, A.G. Excellent outcomes in a geriatric patient with multiple brain metastases undergoing surgical resection with cesium-131 Implantation and stereotactic radiosurgery. Cureus 2017, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuwata, T.; Iwata, T.; Iwanami, T. Napsin a and thyroid transcription factor-1-positive cerebellar tumor with epidermal growth factor receptor mutation. Case Rep. Oncol. 2011, 4, 564–568. [Google Scholar] [CrossRef] [PubMed]
- Hainsworth, J.D.; Spigel, D.R.; Farley, C.; Thompson, D.S.; Shipley, D.L.; Greco, F.A. Phase II trial of bevacizumab and erlotinib in carcinomas of unknown primary site: The minnie pearl cancer research network. J. Clin. Oncol. 2007, 25, 1747–1752. [Google Scholar] [CrossRef]
- Hainsworth, J.D.; Spigel, D.R.; Thompson, D.S.; Murphy, P.B.; Lane, C.M.; Waterhouse, D.M.; Naot, Y.; Greco, F.A. Paclitaxel/carboplatin plus bevacizumab/erlotinib in the first-line treatment of patients with carcinoma of unknown primary site. Oncologist 2009, 14, 1189–1197. [Google Scholar] [CrossRef]
| Contrast-Enhanced Computed Tomography (CT) Scan | Can be performed in emergency, no contraindications (except pregnancy and allergy to contrast medium). Allows visualization of acute bleeding. Less sensitive than MRI (especially for lesions in the posterior fossa, multiple punctate metastases and for leptomeningeal metastases) |
| Contrast-enhanced magnetic resonance (MRI) | Higher resolution then CT scan; needs patient’s collaboration (not suitable in case of psychomotor agitation, claustrophobia). Contraindicated in patients with medical devices that are not compatible (some types of pacemaker, metallic implants, etc.) and if there are contraindications to the contrast medium (allergy, risk of nephrogenic systemic fibrosis, etc.). |
| Diffusion-weighted (DW)-MRI | Useful in differential diagnosis with abscesses: diffusion usually restricted in abscesses and unrestricted in BM. Exception: mucinous BM can show restricted diffusion |
| Gradient echo/other susceptibility-weighted images (SWI) | Useful for the identification of hemosiderin and other blood breakdown products. May improve detection of haemorrhagic BM. |
| Perfusion MRI - cerebral blood volumes (CBVs) | Peri-tumoral CBV lower in BM than in malignant gliomas, while higher in BM than in abscesses |
| MRI spectroscopy (MRS) | Lower choline/creatinine ratios in BM compared to high grade gliomas. |
| 18F-fluordesoxyglucose (FDG)-Positron emission tomography (PET) | Lower sensitivity and specificity than MRI in the detection of BMs. Does not provide enough information for differential diagnosis. Whole body FDG-PET useful to identify the primary tumour or other metastasis (see main text). |
| Squamous Cell K. | CK5/6, CK7, EMA, GFAP |
| Small cells K of the lung | CD56, CK7, TTF1, EMA |
| Lung Adeno-K | CK7, TTF1, Napsin A, EMA, CAM 5.2, CEA, RCC (Variable) |
| Breast Adeno-K | CK5/6 (Variable), CAM 5.2, CK7, GCDFP15, EMA, S100 (variable), CEA, CA 15-3 |
| Endometrial K | CAM 5.2, CK7, CA 125, ER (variable), CA125, CEA |
| Colorectal Adeno-K | CAM 5.2, CK20, CDX2, EMA, CEA, CA 19.9 |
| Stomach Adeno-K | CAM 5.2, CK7, CK20, CDX2, EMA, CEA, CA 19.9 |
| Prostate Adeno-K | PSA, EMA |
| Urothelial K | CK5/6, CK7, CK20, EMA |
| Renal cell K | RCC, EMA, PAX8, Vimentin |
| Melanoma | Vimentin, Melan A, S100, HMB 45 |
| Studies | Histological Diagnosis | Immuno-Histochemical Markers Used | Confirmed Primaries at Follow-Up |
|---|---|---|---|
| Matsunaga et al., 2019 [27] | 42 Adeno-K, 4 squamous-cells, 2 neuroendocrine | NS | NS |
| Mavrakis et al., 2005 [6] | 7 Adeno-K, 2 poorly differentiated malignant epithelial tumours | NS | 2/9 (22%) |
| Drlicek et al., 2004 [39] | Lung 5, colorectum 1, breast 1, kidney 1 | CK (AE1/AE3, 7, 10/13, 18, 20), vimentin, protein S100, TTF-1, and CA 15-3, 19.9, 125, PSA | 0 |
| Bartelt and Lutterbach, 2003 [14] | 15 Lung Adeno-K,4 Squamous-cells, 4 Large-cells, 4 Small-cells, 20 Other | NS | NS |
| Klee et al., 2002 [60] | 14 Adeno-K (12 CK 7 +, none CK 20), 1 carcinoma (no CK 7, CK 20 and CK), 1 melanoma | CK7, CK20, PSA, HCG, CA125 and “antibodies indicating breast or pulmonary primary” | 7/14 (50%) |
| Rudà et al., 2001 [61] | 4 Lung Adeno-K, 1 Squamous-cells, 3 Colon Adeno-K, 1 Pancreatic Adeno-K, 18 no diagnosis | NS | 27/33 (81%) |
| Maesawa et al., 2000 [17] | 10 Adeno-K, 2 Squamous-cells, 1 Clear-cells, 2 Undifferentiated | NS | 4/15 (26.7%) |
| Nguyen et al., 1998 [18] | 31 Adeno-K, 2 small-cells,1 squamous-cells, 4 other, 1 missing | NS | 12/39 (31%) |
| Salvati et al., 1995 [10] | 65 Adeno-K, 10 Squamous-cells, 10 Melanoma, 7 Undifferentiated, 7 other small-cells | NS | 64/100 (64%) |
| Debevec, 1990 [44] | Anaplastic K and adeno-K were most frequent | NS | 47/75 (63%) |
| Merchut, 1989 [7] | 8 Adeno-K + 1 squamous-cells | NS | 47/56 (84%) |
| Chee and Byrnes, 1988 [4] | 5 Adeno-K, 3 anaplastic, 4 squamous-cells, 1 sarcoma, 1 transitional-cells | NS | 35/51 (68%) |
| Eapen et al., 1988 [23] | 9 No diagnosis, 19 Adeno-K, 7 Squamous K, 5 Anaplastic K, 1 large-cells, 1 small-cells, 1 transitional-cells | NS | 11/43 (25%) |
| Zimm et al., 1981 [28] | 14 Adeno-K, 2 squamous | NS | 10/16 (37%) |
| Studies | N of BM- CUP | Treatment of BM | Survival | Local Control | Causes of Death | Determinants of Survival |
|---|---|---|---|---|---|---|
| Gough et al., 2020 [66] | 55 | Surgery | Median survival = 6 m; survival at 1 y = 29.1%; at 5 y = 0. | Median time to recurrence = 6 m | NS | Decreased survival associated with other metastases, older age and ECOG score. No significant difference between CUP Vs known primary tumour. |
| Matsunaga et al., 2019 [27] | 152 | GKRS | Median survival = 6 m; survival at 6 m = 79.3%, at 12 m = 14.9% | Response at 6m: complete = 4.4%, partial = 74.2%, stable = 13.3%, progressive disease = 8.1% | Systemic progression = 67% brain progression = 33%; | Decreased survival associated with higher age, lower KPS score, extracranial MTS, multiple BM |
| Dziggel et al., 2018 [31] | 8 | SRS | Survival at 6 m = 63%; at 12 m = 63%. Median survival time was not reached during the follow up. | Local control 100% at 12 m; freedom from new cerebral lesions at 6 m = 86%, 10 m = 64% | NS | Improved survival associated with male gender, single BM |
| Rades et al., 2018 [38] | 140 | WBRT | Survival at 6 m = 33%; at 12 m = 18%. | Local control: 6 m = 36% 12 m = 24%. | NS | Improved survival associated with ECOG-score, extra-cerebral lesions |
| Han et al., 2016 [25] | 10 | GKRS | Median survival = 35.3 m | Median times to new lesion detection = 4.6 months | Systemic progression = 79%, brain progression = 16%; unrelated cause = 5% | NS. No significant difference between CUP Vs known primary tumour |
| Niranjan et al., 2010 [8] | 29 | SRS | Median survival = 12 m | Local control after 18.6 m = 88.5%. Progression free survival at 6 m = 96.4%, at 12 m = 81.9% | Systemic progression = 90%, brain progression = 10% | Decreased survival associated with location in the brainstem |
| Yamamoto et al., 2009 [36] | 32 | GKRS | Median surviva l = 6.5–7 m | NS | NS | Improved survival associated with n of lesions, tumour volumes, non-symptomatic, well-controlled primary, no extracranial MTS, KPS > 80%, >2 procedures |
| Rades et al., 2007 [32] | 101 | WBRT=Long course (10 × 3 Gy) Vs short-course WBRT (5 × 4 Gy) | Median survival in RPA-I = 7.1 m, in RPA-II = 4.2 m, in RPA-III = 2.3 m | Local control at 6 m: short WBRT = 80%, long WBRT = 50% | NS | Improved survival associated with KPS > 70, no extracranial metastases, RPA-class = 1 |
| D’Ambrosio and Agazzi, 2007 [22]; Agazzi et al., 2004 [19] | 35 | WBRT + Surgery/SRS | Median survival (after diagnosis) = 3.2 m | NS | Systemic progression in most (NS) | No significant difference between CUP Vs known primary. In CUP, no difference if identification of the primary |
| Bartelt and Lutterbach, 2003 [14] | 47 | WBRT | Survival at 3m = 68%, at 6 m = 39%, at 12 m = 26%, at 24 m = 5% | NS | NS | Improved survival associated with KPS > 70 and resection. No significant difference between CUP Vs known primary tumour. |
| Petrovich et al., 2002 [40] | 14 | GKRS | Median survival = 6 m | NS | Systemic progression = 70%, brain progression = 23%, unknown = 7%. | Improved survival associated with KPS > 70, less active systemic disease, total intracranial tumour volume (<3 cm) |
| Yuile and Tran, 2002 [41] | 25 | WBRT | Median survival = 4 m | NS | NS | Improved survival associated with Radiation dose >40 Gy, degree of surgery, primary site (lung) |
| Rudà et al., 2001 [61] | 33 | WBRT + surgery | Median survival = 10 m; Survival at 6 m = 76%, at 12 m = 42%, at 24 m = 15% | NS | NS | Improved survival associated with single BM |
| Hall et al., 2000 [42] | 34 | WBRT | Survival (after diagnosis): at 2 y = 12.3%, at 3 y = 6.6%, at 5 y = 0% | NS | Systemic progression in most (NS) | Improved survival associated with younger age, single of BM, surgical resection, WBRT, CT |
| Maesawa et al., 2000 [17] | 15 | SRS | Median survival = 15 m survival at 2 y = 53.3%, at 3 y = 20% | Crude local tumour control rate = 92.6%. Tumour control rate at 4 y = 91.3% | Systemic progression = 53%, Brain progression = 20%, unknown = 27% | Improved survival associated with BM location (other than brainstem), extracranial disease. Detection of the primary site did not affect survival |
| Lagerwaard et al., 1999 [16] | 102 | Surgery and/or RT | Median survival (after diagnosis) = 5.5 m; Survival at 6 m = 48%, at 1 y = 22%, at 2 y = 10% | NS | Brain progression = 57%. | Improved survival associated with surgery+RT, ECOG, response to steroid treatment, systemic activity, serum LDH, unknown primary, age, number of BM |
| Nguyen et al., 1998 [18] | 27 | WBRT + surgery | Median survival = 13.4 m Survival at 12 m = 56%, at 18 m = 38%, at 5 y = 15%, at 8 y = 12% | 5 y = 72% | Systemic progression in most (NS) | Improved survival associated with gross total resection and RT |
| Nussbaum et al., 1996 [43] | 33 | WBRT + surgery/CT | Median survival (after diagnosis) = 7 m | NS | NS | Improved survival associated with surgery, RT, CT and younger age. |
| Salvati et al., 1995 [10] | 100 | Surgery | Survival at 6 m = 43%, at 1 y = 19% | Intracranial progression/relapse = 60% | Perioperative mortality = 6%; brain progression = 38%; systemic progression = 66% | Improved survival associated with unknown primary, RT, number of MTS in different organs <2 |
| Debevec, 1990 [44] | 75 | WBRT | Median survival = 9.5 m; survival at 1 y = 41%. | NS | Brain progression = 60% | NS |
| Merchut, 1989 [7] | 56 | WBRT/surgery | Survival at 6 m = 55%, at 1 y = 13% | NS | NS | NS |
| Eapen et al., 1988 [23] | 43 | Surgery and/or WBRT | Survival at 6 m = 52%, 12 m = 20% | NS | Brain progression = 68.3%, systemic progression = 31.7% | Improved survival associated with single BM |
| Chevalier et al., 1985 [21] | 67 | Surgery and/or WBRT | Survival (after diagnosis) at 6 m = 44%, at 12 m = 16%, at 24 m = 5% | NS | NS | NS. No significant difference between CUP Vs found primary tumour |
| Yardeni et al., 1984 [45] | 26 | Surgery | Median survival = 3.5 m, survival at 1 y = 19.2%, 2 y = 11.5% | NS | NS | NS. No correlation between survival and age or extracranial metastasis. |
| Zimm et al., 1981 [28] | 16 | Surgery + WBRT/CT 1 | Median survival (after diagnosis) = 7.2 m | NS | Brain progression = 75% | Improved survival associated with younger age, single BM, ambulatory performance status <1, presenting symptoms (headache, personality change, visual disturbances) |
| Ebels and van der Meulen, 1978 [46] | 19 | Surgery | Median survival = 6.8 m | NS | NS | NS |
| Table | Type of Study | Intervention | CUP Inclusion Criteria | BM Inclusion Criteria | Primary end Point |
|---|---|---|---|---|---|
| NCT01540058 | Phase 3 | Experimental: test-guided strategy (primary cancer suspected by “the BioTheranostics Cancer Type ID test” molecular analysis) Vs. Active Comparator: Empiric strategy (Gemcitabine/Cisplatin) | Histopathological confirmed (with IHC): moderately or well-differentiated adenocarcinoma, poorly differentiated adenocarcinoma, undifferentiated carcinoma, squamous-cell carcinoma | Not symptomatic | Progression free survival (death/RECIST criteria) |
| NCT03498521 | Phase 2 Randomized | Experimental: Molecularly Guided Therapy 1 (based on genomic profile) Vs. Active Comparator: platinum-based chemotherapy (Carboplatin or Cisplatin in combination with Gemcitabine or Paclitaxel) | CUP diagnosed according to criteria defined in the 2015 ESMO Guidelines | Previously treated BM without residual disease or leptomeningeal disease | Progression free survival (death/RECIST criteria) |
| NCT03396471 | Phase 2 Single-arm | Pembrolizumab + External Beam Radiation Therapy | CUP after complete negative diagnostic workup | Previously treated BM, stable >4 weeks, no new or enlarging BM, no steroids for > 7 days. No carcinomatous meningitis. | Response rate (irRECIST and RECIST criteria) |
| NCT02721732 | Phase 2 Single-arm | Pembrolizumab | Advanced (unresectable and/or metastatic) solid tumour (including CUP) that has progressed following standard therapies (if available) | Previously treated BM, stable >4 weeks, no new or enlarging BM, no steroids for > 7 days. No carcinomatous meningitis. | Non-progression rate (irRECIST and RECIST criteria) |
| NCT04273061 | Phase 2 Single-arm | Atezolizumab | Incurable solid tumour (including CUP) with whole genome and transcriptome analysis | Asymptomatic, no SRS < 7 days, WBRT<14 days, resection < 28 days, no ongoing corticosteroids. Stable anticonvulsant therapy permitted. | Overall response rate (RECIST criteria) |
| NCT03752333 | Phase 2 Single-arm | Pembrolizumab | CUP after complete negative diagnostic workup. Both first line and previously treated patients. | Previously treated BM, stable >4 weeks, no new or enlarging BM, no steroids for > 7 days. No carcinomatous meningitis. | Overall response rates by (irRECIST and RECIST criteria) |
| NCT02834013 | Phase 2 Non-Randomized Parallel Assignment | Arm I (nivolumab + ipilimumab); Arm II (nivolumab) | Histologically and/or biochemically confirmed rare cancer (including CUP after complete negative diagnostic workup) | Treated >= 28 days, off steroids > 7 days | Overall response rate (RECIST criteria) |
| NCT03391973 | Phase 2 Single-Arm | Pembrolizumab | CUP after complete negative diagnostic workup | Previously treated BM, stable >4 weeks, no new or enlarging BM, no steroids for > 7 days. No carcinomatous meningitis. | Objective response rate (RECIST criteria) |
| NCT04273061 | Phase 2 Parallel Assignment | Atezolizumab | Incurable solid tumour (including CUP) with whole genome and transcriptome analysis | Asymptomatic, no SRS < 7 days, WBRT<14 days, resection < 28 days, no ongoing corticosteroids. Stable anticonvulsant therapy permitted | Objective response rate (RECIST criteria) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balestrino, R.; Rudà, R.; Soffietti, R. Brain Metastasis from Unknown Primary Tumour: Moving from Old Retrospective Studies to Clinical Trials on Targeted Agents. Cancers 2020, 12, 3350. https://doi.org/10.3390/cancers12113350
Balestrino R, Rudà R, Soffietti R. Brain Metastasis from Unknown Primary Tumour: Moving from Old Retrospective Studies to Clinical Trials on Targeted Agents. Cancers. 2020; 12(11):3350. https://doi.org/10.3390/cancers12113350
Chicago/Turabian StyleBalestrino, Roberta, Roberta Rudà, and Riccardo Soffietti. 2020. "Brain Metastasis from Unknown Primary Tumour: Moving from Old Retrospective Studies to Clinical Trials on Targeted Agents" Cancers 12, no. 11: 3350. https://doi.org/10.3390/cancers12113350