The promise of PD-1 inhibitors in gastro-esophageal cancers: microsatellite instability vs. PD-L1
Introduction
Gastric and esophageal cancers are the 3rd and 5th highest causes of cancer mortality worldwide, respectively, resulting in a total of 1.4 million deaths per year (1). In the United States, ~43,000 new cases and ~25,000 deaths from gastro-esophageal cancer are estimated to occur in 2016 (2). Most gastric cancers (GCs) are associated with Helicobacter Pylori (H. Pylori) and Epstein-Barr virus (EBV) infection, with chronic infection from H. Pylori causing about 90% of new cases of noncardiac GC worldwide (3-5). A small percentage of GCs arise from germline mutation in E-Cadherin (CDH1), which is associated with tumors displaying diffuse-type histology (6). Esophageal squamous cell carcinomas (ESCC) comprise 90% of esophageal cancers (ECs) in high-risk areas (including northern Iran, central Asia, and north-central China) and are thought to be related to poor nutritional status, low intake of vegetable and fruits, and high-temperature beverage drinking (1,7-10). Esophageal adenocarcinoma (EAC) is the more prevalent esophageal tumor in the U.S. and Europe, where its incidence has increased significantly in the last 30 years in parallel with obesity and gastro-esophageal reflex disease (11).
The need for identifying effective novel therapies is highlighted by the poor prognosis of patients with advanced gastro-esophageal cancer (survival ~12 months). Multiple randomized trials have shown negative results for agents targeting EGFR, VEGF, MTOR, and hedgehog. To date, only two biologic therapies have been shown to improve overall survival in these patients: trastuzumab, a monoclonal antibody (mAb) targeting human epidermal growth factor 2 (HER2, ERBB2), and ramucirumab, a mAb targeting vascular endothelial growth factor receptor 2 (VEGFR2) (12-17).
Recent studies indicate that immune-modulating therapies may have efficacy in these tumors. A major question is whether molecular subsets can be identified in which these new therapies have increased efficacy. In this review article, we will discuss the available evidence for PD-1/PD-L1 blockade in gastro-esophageal cancers and the potential role of PD-L1 expression and microsatellite instability (MSI) as predictive biomarkers for PD-1/PD-L1 targeted treatment.
PD-1/PD-L1 pathway
The immune system can specifically identify and eliminate tumor cells on the basis of their expression of tumor-specific antigens or molecules induced by cellular stress (18). In this process, known as tumor immune surveillance or immunoediting, the immune system identifies cancerous and/or precancerous cells and eliminates them before they can cause harm. However, if elimination is incomplete, a temporary state of equilibrium can develop between the immune system and the developing tumor. During this period it is envisaged that tumor cells either remain dormant or continue to evolve, accumulating further changes (such as DNA mutations or changes in gene expression) that can modulate the tumor-specific antigens and stress-induced antigens that they express. If the immune response still fails to completely eliminate the tumor, tumor cell variants are selected that are able to resist, avoid, or suppress the antitumor immune response, leading to immune escape.
Tumor infiltration by T cells, particularly cytotoxic T lymphocytes (CTLs), is part of the adaptive antitumor immune response and is believed to represent the elimination or equilibrium phase of immunoediting. The presence of tumor infiltrating lymphocytes (TILs) has been associated with an improved prognosis for a number of different tumor types, including colorectal and GCs (19,20). CTL function is closely regulated by the tumor microenvironment, which consists of cancer cells, inflammatory cells, stromal cells and cytokines (21). The immunosuppressive network in the tumor microenvironment usually drives the CTL/TIL into “exhaustion”, a state of T-cell dysfunction marked by a unique molecular signature and that results in decreased cytokine expression and effector function (22).
Programmed cell death protein 1 (PD-1) pathway is considered an important inhibitory mechanism regulating T-cell exhaustion. PD-1 is expressed on the surface of T cells, B cells, monocytes and nature killer cells (23,24), including in TILs. PD-1 has two main ligands, PD-L1 and PD-L2. PD-L1 is expressed on T cells, B cells, dendritic cells, macrophages, mesenchymal stem cells and bone marrow-derived mast cells, and some non-hematopoietic cells. PD-L2 is expressed on dendritic cells, macrophages, bone marrow-derived mast cells, and resting peritoneal B1 cells (23,24). High expression of PD-L1 and PD-L2 has been detected on tumor cells (23,25). T cell functions are inhibited upon interaction between PD-1 and its ligand PD-L1 and/or PD-L2, which leads to T-cell exhaustion and is proposed as one mechanism underlying a tumor’s ability to evade immune surveillance (26).
PD-L1 expression in gastro-esophageal tumors
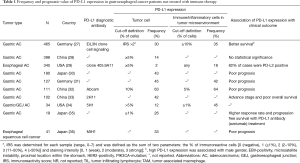
Table 1 shows the results of studies which examined PD-L1 expression in human gastro-esophageal cancer tissues. PD-L1 has been detected in the tumor microenvironment of GCs including tumor cells, stromal and immune cells. The largest gastro-esophageal cancer cohort reported, to date, examined PD-L1 expression in 465 German gastric/gastro-esophageal junction (GEJ) cancer cases by immunohistochemistry (IHC) (antibody E1L3N clone, Cell Signaling) (27). The following expression patterns were found:
Full table
- PD-L1 expression on tumor cell membranes was detected in 30.1% of cases. The percentage of stained tumor cells was generally low [in 90% of the cases, <10% of tumor cells were immunopositive; median 0 (range 0–80%)]. Staining intensity also tended to be low [median 0 (range, 0–3)]. Only a minority of cases showed different staining intensities within the same tumor. The tumor cell immunoreactivity score (IRS), which was the sum of the percentage score and intensity score, was calculated for each case [median 0 (range, 0–7)]. Dichotomized post-hoc by an IRS of 2, 23.9% (111 cases) were classified as positive PD-L1 in tumor cells;
- By contrast, PD-L1 expression in immune cells was found in a majority of cases (88.4%). However, the percentage [median 5% (range 0 to 70%)] and intensity [median 1 (range, 0 to 3)] also tended to be low. For scoring PD-L1 expression in immune cells, cases in which 10% or more immune cells showed any immunostaining were considered positive. Accordingly, 35.5% of cases were considered PD-L1 positive in immune cells;
- PD-1 expression was observed neither in tumor nor in stroma cells. PD-1 diffusely distributed TILs were present in 53.8% of cases. PD-1 expression in TILs was significantly correlated with PD-L1 expression in tumor, stroma and immune cells.
PD-L1 tumor cell expression was detected more often in men and in gastric/GEJ cancers that were EBV-positive, MSI, proximally located, HER2-positive, or PIK3CA-mutated. The association between PD-L1 expression and a covariate was strongest for EBV, with 18 of 20 EBV-positive cases showing PD-L1 expression in tumor cells and 14 of 20 EBV-positive cases showing PD-L1 expression in immune cells.
Likewise, a study of PD-L1 expression and immune infiltration in GCs from Asia (N=398 cases) found that 14% of cases were PD-L1 positive in tumor cells. A sample was considered for PDL1 expression if 5% or more of tumor cells showed membrane positivity. There was correlation between PD-L1 expression and density of TILs.
In both studies, PD-L1 tumor-cell positivity had an association with favorable overall survival that was either statistically significantly (27) or trended toward significance (28). However, other investigations in gastro-esophageal cancer have reported an adverse association between PD-L1 expression and survival (30-33,35,36).
The frequency of PD-L1 expression in EAC was recently reported to be lower than what has been reported to date in GC. PD-1, PD-L1, and PD-L2 expression was analyzed on a tissue microarray (TMA) containing EAC samples from 345 patients. Surprisingly, only 1.7% of cases had PD-L1 positive tumor cells, and only 18% of cases had PD-L1+ staining in inflammatory cells (mostly macrophages). An evaluation of whole-tumor sections from a subset of 45 tumors revealed that the frequency of PD-L1 positive immune cells was somewhat higher [35.6% (16/45) of cases, of which 7/16 were not identified on the TMA], although still lower than that reported in GC. Interestingly, Barrett’s-associated EAC has been reported to have a low frequency of MSI and EBV (see below) (37,38).
On the other hand, 81.6% of EACs were found to be PD-L2 positive in at least one core. While PD-L2 and PD-L1 expression were not mutually exclusive, tumors with PD-L2 expression in all evaluated cores were less likely to possess PD-L1+ immune cells. Both PD-L2+ and PD-L1+ tumors had a higher average number of PD-1+ TILs compared to tumors without PD-L2 or PD-L1 expression. In 15.5% (53/343) of EACs, no PD-L2+, PD-L1+, or PD-1+ cells were observed.
The investigators suggested that the high frequency of PD-L2 expression, in the absence of PD-L1 co-expression, may be due to the fact that EACs develop in a background of chronic inflammation and typically emerge from Barrett’s esophagus (BE), a tissue with a documented Th2-skewed inflammatory state with increased IL4/IL13 expression (39,40). In macrophages and dendritic cells PD-L2 transcription is regulated by IL4/IL13/STAT6 signaling (41,42), raising the possibility that PD-L2 epithelial expression in EAC and BE may result from IL4/IL13 expression (29,43). The therapeutic role of inhibiting PD-L2 is controversial. While some studies show an inhibitory role for PD-L2 (44,45), others suggest that PD-L2 can stimulate T-cell proliferation (46) via a PD-1-receptor independent mechanism, potentially involving a distinct PD-L2 binding partner. Further evaluation of PD-L2 expression in independent EAC cohorts is warranted, including its potential role in predicting response to PD-1 blockade.
The varying rates of PD-L1 positivity and its prognostic value may be due to different patient populations (e.g., race, disease stage), different antibodies, and different cut-points. However, taken together, these studies indicate that PD-1/PD-L1 expression occurs in a subset of tumors, suggesting a molecular target exists for therapeutic inhibition of the PD-1/PD-L1 pathway. In addition, these data suggest that the immune microenvironment of EAC may differ from gastric/GEJ adenocarcinomas, and should be an area of further investigation.
Anti-PD-1/PD-L1 therapy in gastro-esophageal tumors
PD-1/PD-L1 blockade has recently been shown to be a promising treatment in a variety of tumor types (47-50). The PD-1 inhibitors, pembrolizumab and nivolumab, are both immunoglobulin G4 (IgG4) antibodies, which bind to PD-1 to disrupt the interaction between PD-1 and its ligands and thereby impede inhibitory signals in T cells (51,52). Pembrolizumab is FDA-Approved for the treatment of unresectable or metastatic melanoma and for PD-L1 positive metastatic non-small cell lung cancer (NSCLC) (47,53-55). Nivolumab is FDA-approved for the treatment as a single agent or in combination with ipilimumab for the treatment of unresectable or metastatic melanoma, metastatic NSCLC that progresses on or after platinum based chemotherapy, and advanced renal cell carcinoma after anti-angiogenic therapy (49,50,56-62).
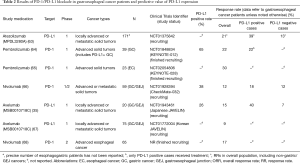
Table 2 shows the results from trials, to date, which examined anti-PD-1/PD-L1 therapy in gastro-esophageal cancer patients. The first reported evaluation of a PD-1/PD-L1 inhibitor in esophageal or GC examined pembrolizumab in PD-L1-positive GC patients. In this study (KEYNOTE-012), 162 patients with advanced gastric/GEJ cancer (recurrent or metastatic) were screened for PD-L1 expression by 22C3 antibody IHC staining. Only patients with distinctive stromal or ≥1% tumor nest cell PD-L1 staining were eligible. A total of 65 patients were considered PD-L1 positive, of which 39 were enrolled onto the trial and treated with pembrolizumab. A majority of patients [66.7% (16/39)] received more than one prior treatment. The overall response rate (ORR) was 22% by central review. After a median follow up of 8.8 months, the 6-month progression free survival (PFS) rate was 24% and 6-month overall survival (OS) rate was 69%. Median time to response was 8 weeks with a median response duration (RD) of 24 weeks. The drug appeared to be well tolerated. Four patients experienced five grade 3–5 drug-related adverse events, including fatigue, decreased appetite, peripheral sensory neuropathy, hypoxia and pneumonitis. One patient died of hypoxia (64).
Full table
As shown in Table 3, the ORR observed for pembrolizumab monotherapy compares favorably to single-agent response rates observed in randomized trials for ramucirumab in the second-line setting (ORR <4%, duration of therapy 8 weeks; REGARD) or regorafenib in the first-/second-line setting (ORR 3%, duration of therapy 8 weeks; INTEGRATE). However, response rates were higher in the RAINBOW study for paclitaxel arm (RR ~17%) and paclitaxel-ramucirumab arm (RR ~28%). In addition, the median PFS and OS observed for pembrolizumab is comparable to those reported in second-line trials studying these other therapies (15,17). While cross-trial comparisons must be viewed with caution, the non-randomized data from KEYNOTE-012 provide proof-of-concept that anti-PD-1 therapy may have activity in advanced GC and warrants further study.

Full table
Nivolumab has also demonstrated single-agent activity. In CheckMate-032, patients with solid tumors were treated with nivolumab with or without ipilimumab. Preliminary results from a subset of patients with advanced gastric or GEJ cancer of any PD-L1 status (n=59) who were treated with nivolumab monotherapy demonstrated an ORR of 12% (1 CR, 6 PRs) and a median RD of 7.1 months (66).
Table 2 also shows available data from studies examining the PD-L1 antibodies avelumab (MSB0010718C), atezolizumab (MPDL3280A), and durvalumab (MEDI4716). In general, RRs in gastro-esophageal cancer patients for these antibodies have ranged from 10% to 30% (35,63,65,67,70-73).
Given the variability of response to PD-1 blockade, research attention has concurrently focused on identifying biomarkers of response so as to select patients who are most likely to benefit. The candidate biomarker studied most extensively is PD-L1 expression in trials utilizing PD-1 blockade.
PD-L1 expression as a predictive marker for PD-1/PD-L1 blockade
At least five large studies have examined PD-L1 expression as a predictive biomarker of anti-PD-1 therapy in patients with advanced carcinoma. A phase 1 study of pembrolizumab monotherapy in 495 NSCLC patients (Keynote-001, NCT10295827), consisting of both squamous and non-squamous histology, showed a correlation between PD-L1 expression and ORR (52). Using the anti–PD-L1 antibody clone 22C3 (Merck), PD-L1 expression was analyzed in the membrane of tumor cells and in intercalated mononuclear inflammatory cells within tumor nests and stroma adjacent to tumor nests. After the study was initiated, membranous PD-L1 expression in at least 50% of tumor cells (proportion score, ≥50%) was selected as the cut-off on the basis of the ease of use and receiver-operating-characteristic (ROC) analysis. ORRs were 45%, 17% and 11% in the overall population with PD-L1 proportion scores of ≥50%, 1–49% and <1%, respectively. Median PFS among patients with a PD-L1 proportion score of at least 50% was 6.3 months (95% CI, 2.9 to 12.5), and was shorter among patients with a proportion score of <50%. Median OS among patients with a PD-L1 proportion score of at least 50% was not reached in the total population (95% CI, 13.7 months to not reached), and was shorter among patients with a lower proportion score. The median duration of response was similar regardless of proportion score. Based on this study results, the FDA approved pembrolizumab for the treatment of advanced NSCLC in October 2015 with diagnostic PD-L1 IHC 22C3 pharmDx test.
The predictive value of PD-L1 expression was also analyzed in squamous vs. non-squamous NSCLC populations separately in two studies. In both studies, patients with advanced NSCLC were randomized to nivolumab or docetaxel in the second-line setting. Identical methodology was used to assess PD-L1 expression in both studies. PD-L1 expression levels were analyzed in pre-treatment (archival or recent) tumor biopsy specimens using a validated automated IHC assay (Epitomics, clone 28-8). Samples were categorized as positive when staining of the tumor-cell membrane (at any intensity) was observed in a pre-specified percentage of cells (1%, 5%, or 10%) in a section that included at least 100 evaluable tumor cells. Despite similar methodology, the conclusions of one study differed from the other.
The non-squamous lung cancer trial (CheckMate-057, N=582) was a randomized, open-label, international phase 3 study of patients with non-squamous NSCLC [93% had adenocarcinoma (AC)] that had progressed during or after platinum-based doublet chemotherapy. Patients were treated with nivolumab (3 mg/kg every 2 weeks) or docetaxel (75 mg/m2 every 3 weeks). The clinical study met its primary endpoint of OS, with a median OS of 12.2 months in the nivolumab group and 9.4 months in the docetaxel group (P=0.002). The response rate was 19% with nivolumab versus 12% with docetaxel (P=0.02). Although median PFS did not favor nivolumab over docetaxel (2.3 vs. 4.2 months, respectively), the 1-year PFS rate was higher with nivolumab than with docetaxel (19% and 8%, respectively).
In this trial, 78% (455/582) of randomized patients had quantifiable PD-L1 expression. The rate of PD-L1 positivity was 54% (123/455) at the 1% cut-point, 40% (181/455) at the 5% cut-point, and 36% (165/455) at the 10% cut-point. Nivolumab was associated with greater efficacy than docetaxel across all end points (RR, PFS, and OS) in subgroups defined according to pre-specified levels of tumor-membrane expression (≥1%, ≥5%, and ≥10%) of PD-L1. As an example, at the 10% cut-point for PD-L1 expression, the RR from nivolumab in PD-L1 positive vs. negative tumors was 37% vs. 11%, whereas docetaxel-treated patients had a RR of ~14% regardless of PD-L1 status. For PFS, among PD-L1 positive tumors, nivolumab-treated patients had a median PFS of 5.0 months, as compared to 3.7 months among docetaxel-treated patients [HR 0.52 (0.37, 0.75)]. By contrast, there was no PFS benefit for nivolumab among PD-L1 negative tumors [median PFS 2.1 months with nivolumab vs. 4.2 months with docetaxel; HR 1.24 (0.96, 1.61)]. Results were similar for OS, as follows: Among PD-L1 positive tumors, nivolumab-treated patients had a median OS of 19.9 months, as compared to 8.0 months among docetaxel-treated patients [HR 0.40 (0.27, 0.58)]. By contrast, there was no OS benefit for nivolumab among PD-L1 negative tumors [median PFS 9.9 months with nivolumab vs. 10.3 months with docetaxel; HR 0.96 (0.74, 1.25)]. Importantly, interaction P values were statistically significant for all PD-L1 expression cut-point levels and clinical endpoints (with the exception of the 1% cut-point for OS), strongly indicating PD-L1 expression was predictive of nivolumab benefit. A caveat is that randomization was not stratified by PD-L1 expression—i.e., the PD-L1 subgroup analysis disrupted randomization and thus is not definitive. The authors indicated that the improved safety profile and durability of responses to nivolumab suggest that it might be a reasonable option for patients regardless of PD-L1 expression.
By contrast, PD-L1 expression was not found to be predictive in a smaller trial of squamous lung cancers (checkmate-017, N=272). In this trial, patients with molecularly unselected stage IIIB or IV cancer who had disease progression after one prior platinum-containing regimen were randomized to nivolumab vs. docetaxel. An OS benefit favoring nivolumab over chemotherapy alone was demonstrated (median OS of 9.2 vs. 6.0 months, respectively). A total of 83% (225/272) of randomized patients had quantifiable PD-L1 expression. The rate of PD-L1 positivity was 53% (119/225) at the 1% cut-point, 36% (81/225) at the 5% cutpoint, and 31% (69/225) at the 10% cut-point. The authors reported that PD-L1 expression was neither prognostic nor predictive of RR, PFS, or OS across all pre-specified expression levels of PD-L1 (1%, 5%, and 10%). While the survival curves suggested a slightly greater benefit from nivolumab for PD-L1 positive tumors as compared to PD-L1 negative tumors [e.g., HR for PFS 0.54 (95% CI 0.32, 0.90)] for the PD-L1 positive group at the 5% cut-point vs. HR 0.75 [(0.52, 1.1) for the PD-L1 negative group], nivolumab prolonged OS as compared to docetaxel in patients with PD-L1 negative tumors—indicating that PD-L1 tumor expression was not a strong predictive marker (49).
It is possible that the difference in results for PD-L1 expression as a predictive marker between the two NSCLC studies may be due to a difference in the immune milieu between squamous vs. non-squamous NSCLCs, which is an area that should be studied further. However, dynamic PD-L1 expression related to the tumor microenvironment (74) and responses observed in patients with low PD-L1 expression levels have raised questions on whether PD-L1 expression is an ideal marker for PD-1 treatment.
In support of this concept, PD-L1 expression was found not to be predictive in a phase 3 trial (CheckMate-025; N=821) of previously treated advanced clear-cell renal-cell carcinoma (RCC). In this trial, patients randomized to receive nivolumab had an OS of 25 months, as compared to 19.6 months for patients in the everolimus arm. PD-L1 expression was not predictive for response across different expression levels. Median OS in the nivolumab arm were 21.8 months and 27.4 months for tumors with PD-L1 expression levels of ≥1% or <1%, respectively. Results for OS were similarly null using a 5% cutpoint of PD-L1 expression (50).
More recent data have indicated that PD-L1 expression was predictive of OS benefit in NSCLC patients treated with an anti-PD-L1 Ab. Advanced NSCLC patients (N=287; both non-squamous and squamous) were randomized to atezolizumab (an anti-PD-L1 Ab) vs. docetaxel in the second-line setting (75). This phase 2 trial met its primary endpoint of OS (HR 0.73 favoring atezolizumab). Moreover, the benefit in OS was limited to patients whose tumors had any PD-L1 expression in either the tumor or in tumor-infiltrating immune cells [HR 0.59 (95% CI 0.40–0.85)]. By contrast, patients with no PD-L1 expression had no benefit in OS [HR 1.04 (95% CI 0.62–1.75)]. A strength of this study, while not large, is that patients were stratified by PD-L1 expression status. PD-L1 expression status also predicted for improved RR and PFS, although less strongly. This therapeutic antibody and candidate predictive biomarkers are currently being examined in larger NSCLC cohorts.
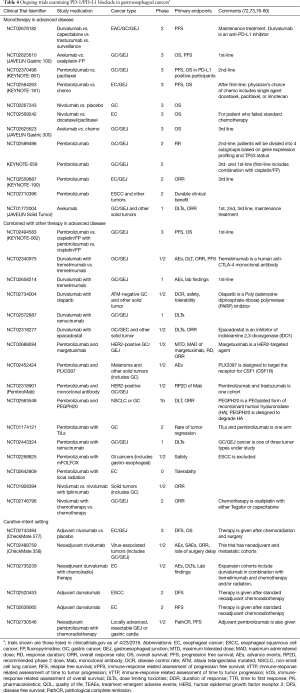
In advanced gastric/GEJ cancer, data are more limited regarding the predictive value of PD-L1 expression. In KEYNOTE-012 (primary results described above), where pembrolizumab monotherapy was administered only to patients with PD-L1 expressing tumors, a trend toward an association between higher levels of PDL1 expression and ORR, PFS, and OS was observed (1-sided P=0.10, 0.16, and 0.12, respectively) (14). Further detail has not been reported. Given the small sample size, and because PD-L1 non-expressing tumors were excluded from KEYNOTE-012, it is currently difficult to say whether PD-L1 expression correlates with pembrolizumab efficacy. The ongoing KEYNOTE-059 study is enrolling patients with advanced gastric/GEJ cancer in both the first- and third-line settings, including both PD-L1 positive and negative tumors and thus may be able to address whether PD-L1 expression is a predictive marker for pembrolizumab. In addition, this question can be further examined in two new phase 3 trials (Table 4): a second-line trial comparing pembrolizumab with paclitaxel (KEYNOTE-061; NCT02370498), which includes tumors of any PD-L1 status; and a new first-line trial comparing pembrolizumab vs. cisplatin/5-FU vs. cisplatin/5-FU plus pembrolizumab, which includes only PD-L1 overexpressing tumors (KEYNOTE-062).
Full table
For nivolumab monotherapy in gastric/GEJ cancer, the CheckMate-032 study (primary results described above) enrolled patients of any PD-L1 status (66). A sample was considered positive if PD-L1 expression was observed on the membrane of tumor cells. These criteria differ from those used in KEYNOTE-012, which considered expression in tumor or immune cell as positive. In CheckMate-032, two different cut-offs for PD-L1 expression were considered positive: 1% or more of tumor cells, and 5% or more. At the 1% cut-off, 38% (15/40) of cases were PD-L1 positive; and at the 5% cut-off, 15% (6/40) were PD-L1 positive. The ORR was numerically higher in PD-L1-positive vs. -negative tumors at both the 1% cut-off (27% in PD-L1 positive tumors vs. 12% in PD-L1 negative tumors) and the 5% cut-off (33% vs. 15%). This trend suggests that PD-L1 expression could be predictive for nivolumab efficacy and is consistent with the data regarding pembrolizumab monotherapy. While intriguing, these data require confirmation in larger cohorts.
Taken together, data from large clinical trial populations of advanced carcinoma (excluding melanoma) suggests that baseline PD-L1 expression in the tumor membrane may have some value in predicting efficacy from PD-1/PD-L1 blockade. However, the data are inconsistent across tumor types, and a potential benefit from checkpoint inhibition has not been ruled out in patients with PD-L1-negative tumors. Data from gastric/GEJ cancer are sparse and similarly suggest that PD-L1 expression may have some predictive value, but to date, has not ruled out benefit for patients whose tumors lack expression. The inconsistency of results may reflect the fact that PD-L1 and PD-1 expression are dynamic markers that change in relation to local cytokines and other factors, and they may be an imperfect surrogate for an “immunogenic” tumor microenvironment that has higher rates of functionally exhausted T cells infiltrating the tumor. In addition, the varying results between studies could be due to differences in antibodies used, definitions of positivity (cut-off percentage, expression in tumor cells vs. infiltrating lymphocytes, membranous vs. cytoplasmic expression, etc.) (81-84). Moving forward, eligibility for trials in gastro-esophageal cancer examining PD-1 blockade do not generally appear to be limited to a particular PD-L1 status, which will allow the ability to address the question of its predictive value more definitively.
Mismatch repair (MMR) deficiency and hypermutation in gastro-esophageal tumors
Deficiencies in the DNA MMR system cause errors during DNA replication, which in turn give rise to MSI. Microsatellites, which are simple repeat sequences of 1 to 6 base pairs (also known as short tandem repeats), are particularly prone to DNA replication errors (85). MSI manifests as small increases or decreases (‘‘instability’’) in the number of repeats in microsatellites throughout the genome (85). MMR proteins including MLH1, PMS2, MSH2 and MSH6 can form heterodimers to correct these mismatches. Mutations in any of these MMR genes, as well as in EpCAM (which leads to loss of MSH2 expression), can cause truncated protein products that result in tumorigenesis (86). These unrepaired alterations contribute to carcinogenesis along a distinct pathway (the MSI pathway) that differs from the chromosomal instability pathway. About 15% colorectal cancers harbor MMR defects (deficient MMR, dMMR) (87,88). Among these cases, about 20% are caused by Lynch syndrome which include germline mutations in MMR genes (MLH1, MSH2, MSH6, and PMS2) while the majority of cases (80%) are caused by sporadic hypermethylation of the MLH1 gene promoter (86-91).
Deficient MMR results in the incorporation of mismatched nucleotides, with MMR-deficient colorectal cancer cells exhibiting 10–100 times the number of somatic mutations as those with proficient MMR (92-94). MSI-high status in colorectal cancer is associated with improved stage-specific prognosis although its predictive value is controversial (95-99). It has been observed that frameshift-induced neopeptides in patients with MSI-high colorectal cancer are effectively recognized by the immune system. The better outcome of these patients is partly related to the high infiltration of activated CD8+ cytotoxic lymphocytes (CTL) and T helper 1 cells (Th1) (19,100-102).
MSI occurs in 10–39% of gastro-esophageal cancers. MSI in GC is associated with old age at onset, antral location, differentiated type, distinct mucinous or medullary histological patterns and reduced lymph node metastasis (103,104). In the largest studies, which were from Korea and examined five MSI markers, ~9% of GCs were MSI-H (MSI detected by more than one marker) (105,106). In GC patients from Europe, MSI was detected in 8–16% of cases (107,108). MSI-high GCs have been found to be associated with a denser accumulation of TILs and significantly associated with improved patient survival compared with MSI-stable/-low tumors (106).
MSI-high gastric tumors appear to have a significantly higher somatic mutation load (hypermutated). The most comprehensive molecular characterization of gastric AC was performed by the Cancer Genome Atlas (TCGA) and examined 295 paired gastric AC cases using six molecular platforms (array-based somatic copy number analysis, whole-genome sequencing, array-based DNA methylation profiling, mRNA sequencing, microRNA sequencing and reverse-phase protein array). A new molecular classification describing four subtypes was proposed: MSI, EBV, genomically stable, chromosomal instable (CIN). Data from the MSI and EBV subgroups appeared to have greatest relevance for immune checkpoint treatment. MSI tumors appeared to have a significantly higher somatic mutation load (hypermutated). Genes mutated within MSI tumors revealed common alterations in major histocompatibility complex class I genes, including Beta2 microglobulin (B2M) and HLA-B. B2M mutations in colorectal cancers and melanoma result in loss of expression of HLA class 1 complexes (109), suggesting these events benefit hypermutated tumors by reducing antigen presentation to the immune system. In addition, elevated mutations were found in genes encoding targetable oncogenic signaling proteins. EBV tumors were enriched with PIK3CA mutations and extreme DNA hypermethylation. Interestingly, EBV tumors also showed amplification and/or expression of PD-L1 and PD-L2, providing rationale for testing immune checkpoint inhibitors in EBV-positive GC. Nivolumab monotherapy is currently being evaluated in a study of virus-associated tumors, including gastro-esophageal cancer (CheckMate358; NCT02488759).
MSI was detected in 6.6% (5/76) of patients with Barrett’s-associated EAC, with the presence of MSI limited to AC cells, and not in adjacent Barrett’s or normal epithelia (37,38). MSI has been less well studied in ESCC, although it has been detected in a subset of patients (110-112). Differences in the precise definition of MSI/MMR and test methods may account for some of these reported incidence differences (108).
Predictive value of MSI in PD-1/PD-L1 blockade
A direct correlation between MMR deficiency and an improved efficacy of PD-1/PD-L1 inhibition was recently suggested in a small study examining patients with advanced colorectal cancer (CRC) and non-CRC gastrointestinal tumors treated with pembrolizumab monotherapy. MSI was tested using the Promega system. The immune-related objective response rate (RR) was 40% (4/10) in MMR-deficient CRCs, as compared to 0% (0/18) in MMR-proficient CRCs. Patients with MMR-deficient non-CRC had responses similar to those of patients with MMR-deficient CRC [immune-related objective RR, 71% (5 of 7 patients)]. The median PFS and OS were not reached in the cohort with MMR-deficient CRC but were 2.2 and 5.0 months, respectively, in the cohort with MMR-proficient CRC (HR for disease progression or death =0.10; P<0.001; and HR for death, 0.22; P=0.05). Whole-exome sequencing revealed a mean of 1,782 somatic mutations per tumor in MMR-deficient tumors, as compared with 73 in MMR-proficient tumors (P=0.007), and high somatic mutation loads were significantly associated with prolonged PFS. A mean of 578 potential mutation-associated neoantigens were detected form dMMR tumors while only 21 such neoantigens were detected in MMR-proficient tumors. CD8 and PD-L1 IHC staining was not significantly associated with PFS or OS (94). Interestingly, in the cohort with dMMR non-CRC, all patients with sporadic tumors (n=6) responded to treatment, whereas only 3 of 11 patients with Lynch syndrome responded (94).
A subsequent report by the same group focused attention on dMMR non-CRC patients (66). Twenty-one patients with MMR-deficient tumors were given pembrolizumab (10 mg/kg intravenously every 2 weeks); the primary tumor was reported for 17 patients: ampullary (N=4), pancreas (N=4), biliary (N=3), small bowel (N=3), and gastric (N=3) cancers. For the ten evaluable patients whose results were reported at the 2016 ASCO GI meeting, ORR was 50% (5/10), disease control rate was 70% (7/10), and median OS was 21 months. After a median follow up of 7.6 months, the median duration of response (DOR) (range, 5.5 to 17+ months) and PFS were not reached. Further study of this agent in an expanded cohort is ongoing.
These preliminary data raise the hypothesis that MMR deficiency and somatic mutation burden in gastro-esophageal and other cancers could be a potential marker to predict efficacy from PD-1/PD-L1 treatment. As detected by whole genome or whole exome sequencing, gastric or esophageal cancers have among the highest somatic mutation rates among malignancies, with a median >4 mutations per megabase (MB) observed in each tumor type (113,114). As comparison, melanoma and squamous lung cancers have been reported to have the highest mutational load (10 or more mutations per MB), and pancreatic cancers have a lower rate (<1 mutation per MB).
Table 4 shows studies in the gastro-esophageal cancers are currently ongoing by using compounds target the PD-1 pathway by either antagonizing PD-1 (pembrolizumab, nivolumab) or PD-L1 (atezolizumab).
Preliminary data in other cancers suggest that tumors with high rates of somatic mutations (i.e., sun-exposed cutaneous melanoma, NSCLC in smokers, and microsatellite unstable colorectal carcinomas) have a higher chance of benefiting from immune checkpoint blockade than tumors with lower rates of somatic mutations (94,115,116). Exome sequencing in NSCLC patients treated with pembrolizumab showed that significantly elevated nonsynonymous mutation burden was strongly associated with clinical benefit from pembrolizumab treatment. In this study, the confirmed tumor RR (63% vs. 0%) and median PFS (14.5 vs. 3.7 months) was higher in patients (n=16) with a high vs. low nonsynonymous burden (with the cut-off at the median of 209 mutations per tumor). The PFS finding was confirmed in a validation cohort (n=18). In addition, the quantity of predicted neoantigens per tumor (a measure of immunogenicity) was found to correlate with mutation burden, and a high candidate neoantigen burden was associated with improved PFS. The molecular smoking signature, but not the patient self-reported smoking history, was correlated with treatment efficacy. In this trial, most patients had some degree of PD-L1 expression, limiting the ability to determine associations between mutation burden and PD-L1 expression. However, it appeared that mutation burden was able to distinguish patients who did and did not experience durable clinical benefit (defined as response lasting >6 months), even among those who had some degree of PD-L1 expression. Although this study had a limited sample size, these preliminary data raise the possibility that nonsynonymous mutation burden could serve as a potential biomarker for PD-1 blockade that can provide further predictive information beyond PD-L1 expression (116).
Future areas: checkpoint inhibition combined with chemo(radiotherapy)
Given that cancer cells evade the human immune system using different mechanisms, it is rational to consider combining PD-1/PD-L1 blockade with other therapies. Table 4 shows a sample of ongoing trials examining combination therapy.
Dual immune checkpoint blockade
Cytotoxic T-lymphocyte antigen 4 (CTLA-4) is a T-cell receptor similar to T-cell co-stimulatory protein CD28. Naïve T cells (except regulatory T cells) lack CTLA-4 expression. Upon activation, CTLA-4 becomes expressed on activated CD4+ and CD8+ T cells and competes with CD28 to bind with its ligands, B7-1 (CD80) and B7-2 (CD86). In CD4+ helper T cells, CTLA-4 activation down-regulates the T cell activity while in the CD4+ T regulatory cells, CTLA-4 activation up-regulates the T cell function which leads to termination of the immune response (117,118). Dual immune checkpoint blockade combining anti-CTLA-4 and anti-PD-1 treatment may enhance antitumor effects by targeting different mechanism of T-cell activation. Nivolumab combined with ipilimumab, a CTLA-4 antibody, showed better objective response and progression-free survival in both BRAF wild-type and mutated patients with advanced melanoma (119). Currently, clinical study of dual immune checkpoint blockade in gastro-esophageal cancers is undergoing (NCT01928394).
Combined with anti-vascular endothelial growth factor (VEGF/VEGFR) agents
High levels of VEGF may impede dendritic cell functions and VEGF-targeted treatment may enhance anti-tumor immune response by lowering the level of VEGF (120). The combination of the VEGF antibody, bevacizumab, with interferon alpha showed better survival compared with interferon-alpha monotherapy in patients with metastatic renal cell carcinoma (121), suggesting that the combination of VEGF and PD-1/PD-L1 targeted therapies merit further study. The NCT02443324 study is recruiting patients with locally advanced unresectable or metastatic gastric/GEJ cancer to evaluate the combination of nivolumab with ramucirumab.
With conventional chemotherapy and/or irradiation in curative settings
The possibility that irradiation and/or chemotherapy can induce neoantigen presentation and upregulate PD-L1 expression has been previously described (122-125). The combination of PD-1/PD-L1 blockade with chemotherapy or radiation treatment to enhance immune response has shown early promising results in animal models, and trials examining these combinations in human cancer are currently underway (NCT02268825, NCT02642809). At our institution, we will soon open a non-randomized trial in locally advanced resectable GEJ AC studying the combination of neoadjuvant pembrolizumab with concurrent carboplatin/paclitaxel-based chemoradiation followed by surgery and adjuvant pembrolizumab (NCT02730546).
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Torre LA, Bray F, Siegel RL, et al. Global cancer statistics 2012. CA Cancer J Clin 2015;65:87-108. [Crossref] [PubMed]
- Siegel RL, Miller KD, Jemal A. Cancer statistics 2016. CA Cancer J Clin 2016;66:7-30. [Crossref] [PubMed]
- Uemura N, Okamoto S, Yamamoto S, et al. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med 2001;345:784-9. [Crossref] [PubMed]
- Plummer M, Franceschi S, Vignat J, et al. Global burden of gastric cancer attributable to Helicobacter pylori. Int J Cancer 2015;136:487-90. [Crossref] [PubMed]
- Thompson MP, Kurzrock R. Epstein-Barr virus and cancer. Clin Cancer Res 2004;10:803-21. [Crossref] [PubMed]
- Richards FM, McKee SA, Rajpar MH, et al. Germline E-cadherin gene (CDH1) mutations predispose to familial gastric cancer and colorectal cancer. Hum Mol Genet 1999;8:607-10. [Crossref] [PubMed]
- Tran T, Spechler SJ, Richardson P, et al. Fundoplication and the risk of esophageal cancer in gastroesophageal reflux disease: a Veterans Affairs cohort study. Am J Gastroenterol 2005;100:1002-8. [Crossref] [PubMed]
- Islami F, Boffetta P, Ren JS, et al. High-temperature beverages and foods and esophageal cancer risk--a systematic review. Int J Cancer 2009;125:491-524. [Crossref] [PubMed]
- Islami F, Malekshah AF, Kimiagar M, et al. Patterns of food and nutrient consumption in northern Iran, a high-risk area for esophageal cancer. Nutr Cancer 2009;61:475-83. [Crossref] [PubMed]
- Wu M, Liu AM, Kampman E, et al. Green tea drinking, high tea temperature and esophageal cancer in high- and low-risk areas of Jiangsu Province, China: a population-based case-control study. Int J Cancer 2009;124:1907-13. [Crossref] [PubMed]
- Spechler SJ. Barrett esophagus and risk of esophageal cancer: a clinical review. Spechler SJ. JAMA 2013;310:627-36. [Crossref] [PubMed]
- Yoon HH, Shi Q, Sukov WR, et al. Association of HER2/ErbB2 expression and gene amplification with pathologic features and prognosis in esophageal adenocarcinomas. Clin Cancer Res 2012;18:546-54. [Crossref] [PubMed]
- Yoon HH, Sukov WR, Shi Q, et al. HER-2/neu gene amplification in relation to expression of HER2 and HER3 proteins in patients with esophageal adenocarcinoma. Cancer 2014;120:415-24. [Crossref] [PubMed]
- Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 2010;376:687-97. [Crossref] [PubMed]
- Fuchs CS, Tomasek J, Yong CJ, et al. Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): an international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet 2014;383:31-9. [Crossref] [PubMed]
- Shitara K, Muro K, Shimada Y, et al. Subgroup analyses of the safety and efficacy of ramucirumab in Japanese and Western patients in RAINBOW: a randomized clinical trial in second-line treatment of gastric cancer. Gastric Cancer 2016;19:927-38. [Crossref] [PubMed]
- Wilke H, Muro K, Van Cutsem E, et al. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol 2014;15:1224-35. [Crossref] [PubMed]
- Swann JB, Smyth MJ. Immune surveillance of tumors. J Clin Invest 2007;117:1137-46. [Crossref] [PubMed]
- Fridman WH, Dieu-Nosjean MC, Pagès F, et al. The immune microenvironment of human tumors: general significance and clinical impact. Cancer Microenviron 2013;6:117-22. [Crossref] [PubMed]
- Fridman WH. The immune microenvironment as a guide for cancer therapies. Oncoimmunology 2012;1:261-2. [Crossref] [PubMed]
- Osińska I, Domagała-Kulawik J. Bronchoalveolar lavage in lung cancer--diagnostic value and assessment of the anti-cancer immune response. Postepy Hig Med Dosw (Online) 2013;67:1119-27. [Crossref] [PubMed]
- Jiang HB, Yang TJ, Lu P, et al. Gene expression profiling of gastric cancer. Eur Rev Med Pharmacol Sci 2014;18:2109-15. [PubMed]
- McDermott DF, Atkins MB. PD-1 as a potential target in cancer therapy. Cancer Med 2013;2:662-73. [PubMed]
- Keir ME, Butte MJ, Freeman GJ, et al. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol 2008;26:677-704. [Crossref] [PubMed]
- Ahmadzadeh M, Johnson LA, Heemskerk B, et al. Tumor antigen-specific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired. Blood 2009;114:1537-44. [Crossref] [PubMed]
- Wurz GT, Kao CJ, DeGregorio MW. Novel cancer antigens for personalized immunotherapies: latest evidence and clinical potential. Ther Adv Med Oncol 2016;8:4-31. [PubMed]
- Böger C, Behrens HM, Mathiak M, et al. PD-L1 is an independent prognostic predictor in gastric cancer of Western patients. Oncotarget 2016;7:24269-83. [PubMed]
- Geng R, Dai C, Wong A, et al. Prognostic significance of tumor infiltrating immune cells and PD-L1 expression in gastric carcinoma in Chinese patients. J Clin Oncol 2015;33:abstr 4042.
- Derks S, Nason KS, Liao X, et al. Epithelial PD-L2 Expression Marks Barrett's Esophagus and Esophageal Adenocarcinoma. Cancer Immunol Res 2015;3:1123-9. [Crossref] [PubMed]
- Tanaka H, Tamura T, Kimura K, et al. Sakurai K, Toyokawa T, Amano R, et al. Association of the immune checkpoint molecule expression with neutrophil-lymphocyte ratio in patients with gastric cancer: A retrospective study. J Clin Oncol 2016;34:abstr 48.
- Schloesser HA, Drebber U, Thelen M, et al. Comprehensive characterization of PDL-1 and CTLA-4 in gastric cancer. J Clin Oncol 2015;33:abstr 4056.
- Hou J, Yu Z, Xiang R, et al. Correlation between infiltration of FOXP3+ regulatory T cells and expression of B7-H1 in the tumor tissues of gastric cancer. Exp Mol Pathol 2014;96:284-91. [Crossref] [PubMed]
- Wu C, Zhu Y, Jiang J, et al. Immunohistochemical localization of programmed death-1 ligand-1 (PD-L1) in gastric carcinoma and its clinical significance. Acta Histochem 2006;108:19-24. [Crossref] [PubMed]
- Kelly RJ, Thompson E, Zahurak M, et al. Adaptive immune resistance in gastro-esophageal cancer: Correlating tumoral/stromal PDL1 expression with CD8+ cell count. J Clin Oncol 2015;33:abstr 4031.
- Nishina T, Shitara K, Iwasa S, et al. Safety, PD-L1 expression, and clinical activity of avelumab (MSB0010718C), an anti-PD-L1 antibody, in Japanese patients with advanced gastric or gastroesophageal junction cancer. J Clin Oncol 2016;34:abstr 168.
- Ohigashi Y, Sho M, Yamada Y, et al. Clinical significance of programmed death-1 ligand-1 and programmed death-1 ligand-2 expression in human esophageal cancer. Clin Cancer Res 2005;11:2947-53. [Crossref] [PubMed]
- Farris AB 3rd, Demicco EG, Le LP, et al. Clinicopathologic and molecular profiles of microsatellite unstable Barrett Esophagus-associated adenocarcinoma. Am J Surg Pathol 2011;35:647-55. [Crossref] [PubMed]
- Pandilla R, Kotapalli V, Gowrishankar S, et al. Distinct genetic aberrations in oesophageal adeno and squamous carcinoma. Eur J Clin Invest 2013;43:1233-9. [Crossref] [PubMed]
- Fitzgerald RC, Abdalla S, Onwuegbusi BA, et al. Inflammatory gradient in Barrett's oesophagus: implications for disease complications. Gut 2002;51:316-22. [Crossref] [PubMed]
- Moons LM, Kusters JG, Bultman E, et al. Barrett's oesophagus is characterized by a predominantly humoral inflammatory response. J Pathol 2005;207:269-76. [Crossref] [PubMed]
- Lesterhuis WJ, Punt CJ, Hato SV, et al. Platinum-based drugs disrupt STAT6-mediated suppression of immune responses against cancer in humans and mice. J Clin Invest 2011;121:3100-8. [Crossref] [PubMed]
- Loke P, Allison JP. PD-L1 and PD-L2 are differentially regulated by Th1 and Th2 cells. Proc Natl Acad Sci U S A 2003;100:5336-41. [Crossref] [PubMed]
- Gao J, Wu Y, Su Z, et al. Infiltration of alternatively activated macrophages in cancer tissue is associated with MDSC and Th2 polarization in patients with esophageal cancer. PLoS One 2014;9:e104453. [Crossref] [PubMed]
- Latchman Y, Wood CR, Chernova T, et al. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat Immunol 2001;2:261-8. [Crossref] [PubMed]
- Zhang Y, Chung Y, Bishop C, et al. Regulation of T cell activation and tolerance by PDL2. Proc Natl Acad Sci U S A 2006;103:11695-700. [Crossref] [PubMed]
- Wang S, Bajorath J, Flies DB, et al. Molecular modeling and functional mapping of B7-H1 and B7-DC uncouple costimulatory function from PD-1 interaction. J Exp Med 2003;197:1083-91. [Crossref] [PubMed]
- Robert C, Ribas A, Wolchok JD, et al. Anti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: a randomised dose-comparison cohort of a phase 1 trial. Lancet 2014;384:1109-17. [Crossref] [PubMed]
- Topalian SL, Sznol M, McDermott DF, et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol 2014;32:1020-30. [Crossref] [PubMed]
- Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:123-35. [Crossref] [PubMed]
- Motzer RJ, Escudier B, McDermott DF, et al. Nivolumab versus Everolimus in Advanced Renal-Cell Carcinoma. N Engl J Med 2015;373:1803-13. [Crossref] [PubMed]
- Wang C, Thudium KB, Han M, et al. In vitro characterization of the anti-PD-1 antibody nivolumab, BMS-936558, and in vivo toxicology in non-human primates. Cancer Immunol Res 2014;2:846-56. [Crossref] [PubMed]
- Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med 2015;372:2018-28. [Crossref] [PubMed]
- Robert C, Schachter J, Long GV, et al. Pembrolizumab versus Ipilimumab in Advanced Melanoma. N Engl J Med 2015;372:2521-32. [Crossref] [PubMed]
- Ribas A, Puzanov I, Dummer R, et al. Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): a randomised, controlled, phase 2 trial. Lancet Oncol 2015;16:908-18. [Crossref] [PubMed]
- FDA 2016 [cited 2016 04/18/2016]. Available online: http://www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm?fuseaction=Search.Label_ApprovalHistory#apphist
- FDA 2016. Available online: http://www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm?fuseaction=Search.Label_ApprovalHistory
- Gettinger SN, Horn L, Gandhi L, et al. Overall Survival and Long-Term Safety of Nivolumab (Anti-Programmed Death 1 Antibody, BMS-936558, ONO-4538) in Patients With Previously Treated Advanced Non-Small-Cell Lung Cancer. J Clin Oncol 2015;33:2004-12. [Crossref] [PubMed]
- Weber JS, D'Angelo SP, Minor D, et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol 2015;16:375-84. [Crossref] [PubMed]
- Hodi FS, Postow MA, Chesney JA, et al. Clinical response, progression-free survival (PFS), and safety in patients (pts) with advanced melanoma (MEL) receiving nivolumab (NIVO) combined with ipilimumab (IPI) vs IPI monotherapy in CheckMate 069 study. J Clin Oncol 2015;33:abstr 9004.
- Rizvi NA, Mazières J, Planchard D, et al. Activity and safety of nivolumab, an anti-PD-1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non-small-cell lung cancer (CheckMate 063): a phase 2, single-arm trial. Lancet Oncol 2015;16:257-65. [Crossref] [PubMed]
- Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:1627-39. [Crossref] [PubMed]
- Larkin J, Hodi FS, Wolchok JD. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N Engl J Med 2015;373:1270-1. [Crossref] [PubMed]
- Herbst RS, Gordon MS, Fine GD, et al. A study of MPDL3280A, an engineered PD-L1 antibody in patients with locally advanced or metastatic tumors. J Clin Oncol 2013;31:abstr 3000.
- Bang YJ, Chung HC, Shankaran V, et al. Relationship between PD-L1 expression and clinical outcomes in patients with advanced gastric cancer treated with the anti-PD-1 monoclonal antibody pembrolizumab (MK-3475) in KEYNOTE-012. J Clin Oncol 2015;33:abstr 4001.
- Doi T, Piha-Paul SA, Jalal SI, et al. Updated results for the advanced esophageal carcinoma cohort of the phase Ib KEYNOTE-028 study of pembrolizumab (MK-3475). J Clin Oncol 2016;34:abstr 7.
- Le DT, Uram JN, Wang H, et al. PD-1 blockade in mismatch repair deficient non-colorectal gastrointestinal cancers. J Clin Oncol 2016;34:abstr 195.
- Chung HC, Arkenau HT, Wyrwicz L, et al. Safety, PD-L1 expression, and clinical activity of avelumab (MSB0010718C), an anti-PD-L1 antibody, in patients with advanced gastric or gastroesophageal junction cancer. J Clin Oncol 2016;34:abstr 167.
- Kojima T, Hara H, Yamaguchi K, et al. Phase II study of nivolumab (ONO-4538/BMS-936558) in patients with esophageal cancer: Preliminary report of overall survival. J Clin Oncol 2016;34:abstr TPS175.
- Pavlakis N, Sjoquist KM, Tsobanis E, et al. INTEGRATE: A randomized phase II double-blind placebo-controlled study of regorafenib in refractory advanced oesophagogastric cancer (AOGC)—A study by the Australasian Gastrointestinal Trials Group (AGITG), first results. J Clin Oncol 2015;33:abstr 9.
- Horn L, Lawrence DP, Rost S, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 2014;515:563-7. [Crossref] [PubMed]
- Segal NH, Antonia SJ, Brahmer JR, et al. Preliminary data from a multi-arm expansion study of MEDI4736, an anti-PD-L1 antibody. J Clin Oncol 2014;32:abstr 3002.
- ClinicalTrials.gov. Esophageal Cancer PD-1 2016 [3/11/2016]. Available online: https://clinicaltrials.gov/ct2/results?term=esophageal+cancer+PD-1&Search=Search
- ClinicalTrials.gov. Gastric Cancer PD-1 2016 [3/11/2016]. Available online: https://clinicaltrials.gov/ct2/results?term=gastric+cancer+PD-1&Search=Search
- Taube JM, Anders RA, Young GD, et al. Colocalization of inflammatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci Transl Med 2012;4:127ra37. [Crossref] [PubMed]
- Fehrenbacher L, Spira A, Ballinger M, et al. Lancet 2016;387:1837-46. [Crossref] [PubMed]
- ClinicalTrials.gov. Pembrolizumab, Combination Chemotherapy, and Radiation Therapy Before Surgery in Treating Adult Patients With Locally Advanced Gastroesophageal Junction or Gastric Cardia Cancer That Can Be Removed by Surgery [04/11/2016]. Available online: https://clinicaltrials.gov/ct2/show/NCT02730546
- ClinicalTrials.gov 2016 [4/23/2016]. Available online: https://clinicaltrials.gov/ct2/results?term=nivolumab+gastric&Search=Search
- ClinicalTrials.gov 2016 [04/22/2016]. Available online: https://clinicaltrials.gov/ct2/results?term=avelumab+gastric&Search=Search
- clinicalTrials.gov 2016 [4/25/2016]. Available online: https://clinicaltrials.gov/ct2/results?term=MEDI4736+gastric&Search=Search
- ClinicalTrials.gov 2016. Available online: https://clinicaltrials.gov/ct2/results?term=durvalumab+esophageal&Search=Search
- Shukuya T, Carbone DP. Predictive Markers for the Efficacy of Anti-PD-1/PD-L1 Antibodies in Lung Cancer. J Thorac Oncol 2016;11:976-88. [Crossref] [PubMed]
- Teixidó C, González-Cao M, Karachaliou N, et al. Predictive factors for immunotherapy in melanoma. Ann Transl Med 2015;3:208. [PubMed]
- Teixidó C, Karachaliou N, González-Cao M, et al. Erratum to Assays for predicting and monitoring responses to lung cancer immunotherapy. Cancer Biol Med 2015;12:259. [PubMed]
- Teixidó C, Karachaliou N, González-Cao M, et al. Assays for predicting and monitoring responses to lung cancer immunotherapy. Cancer Biol Med 2015;12:87-95. [PubMed]
- Geiersbach KB, Samowitz WS. Microsatellite instability and colorectal cancer. Arch Pathol Lab Med 2011;135:1269-77. [Crossref] [PubMed]
- Ligtenberg MJ, Kuiper RP, Chan TL, et al. Heritable somatic methylation and inactivation of MSH2 in families with Lynch syndrome due to deletion of the 3' exons of TACSTD1. Nat Genet 2009;41:112-7. [Crossref] [PubMed]
- Lynch HT, de la Chapelle A. Hereditary colorectal cancer. N Engl J Med 2003;348:919-32. [Crossref] [PubMed]
- Aaltonen LA, Salovaara R, Kristo P, et al. Incidence of hereditary nonpolyposis colorectal cancer and the feasibility of molecular screening for the disease. N Engl J Med 1998;338:1481-7. [Crossref] [PubMed]
- Hampel H, Frankel WL, Martin E, et al. Screening for the Lynch syndrome (hereditary nonpolyposis colorectal cancer). N Engl J Med 2005;352:1851-60. [Crossref] [PubMed]
- Halvarsson B, Anderson H, Domanska K, et al. Clinicopathologic factors identify sporadic mismatch repair-defective colon cancers. Am J Clin Pathol 2008;129:238-44. [Crossref] [PubMed]
- Hendriks YM, de Jong AE, Morreau H, et al. Diagnostic approach and management of Lynch syndrome (hereditary nonpolyposis colorectal carcinoma): a guide for clinicians. CA Cancer J Clin 2006;56:213-25. [Crossref] [PubMed]
- Timmermann B, Kerick M, Roehr C, et al. Somatic mutation profiles of MSI and MSS colorectal cancer identified by whole exome next generation sequencing and bioinformatics analysis. PLoS One 2010;5:e15661. [Crossref] [PubMed]
- Cancer Genome Atlas Network. Comprehensive molecular characterization of human colon and rectal cancer. Nature 2012;487:330-7. [Crossref] [PubMed]
- Le DT, Uram JN, Wang H, et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N Engl J Med 2015;372:2509-20. [Crossref] [PubMed]
- Goldstein J, Tran B, Ensor J, et al. Multicenter retrospective analysis of metastatic colorectal cancer (CRC) with high-level microsatellite instability (MSI-H). Ann Oncol 2014.1032-8. [Crossref] [PubMed]
- Popat S, Hubner R, Houlston RS. Systematic review of microsatellite instability and colorectal cancer prognosis. J Clin Oncol 2005;23:609-18. [Crossref] [PubMed]
- Ribic CM, Sargent DJ, Moore MJ, et al. Tumor microsatellite-instability status as a predictor of benefit from fluorouracil-based adjuvant chemotherapy for colon cancer. N Engl J Med 2003;349:247-57. [Crossref] [PubMed]
- Sinicrope FA, Foster NR, Thibodeau SN, et al. DNA mismatch repair status and colon cancer recurrence and survival in clinical trials of 5-fluorouracil-based adjuvant therapy. J Natl Cancer Inst 2011;103:863-75. [Crossref] [PubMed]
- Webber EM, Kauffman TL, O'Connor E, et al. Systematic review of the predictive effect of MSI status in colorectal cancer patients undergoing 5FU-based chemotherapy. BMC Cancer 2015;15:156. [Crossref] [PubMed]
- Schwitalle Y, Kloor M, Eiermann S, et al. Immune response against frameshift-induced neopeptides in HNPCC patients and healthy HNPCC mutation carriers. Gastroenterology 2008;134:988-97. [Crossref] [PubMed]
- Yoon HH, Orrock JM, Foster NR, et al. Prognostic impact of FoxP3+ regulatory T cells in relation to CD8+ T lymphocyte density in human colon carcinomas. PLoS One 2012;7:e42274. [Crossref] [PubMed]
- Galon J, Costes A, Sanchez-Cabo F, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science 2006;313:1960-4. [Crossref] [PubMed]
- Leung SY, Yuen ST, Chung LP, et al. hMLH1 promoter methylation and lack of hMLH1 expression in sporadic gastric carcinomas with high-frequency microsatellite instability. Cancer Res 1999;59:159-64. [PubMed]
- dos Santos NR, Seruca R, Constância M, et al. Microsatellite instability at multiple loci in gastric carcinoma: clinicopathologic implications and prognosis. Gastroenterology 1996;110:38-44. [Crossref] [PubMed]
- An JY, Kim H, Cheong JH, et al. Microsatellite instability in sporadic gastric cancer: its prognostic role and guidance for 5-FU based chemotherapy after R0 resection. Int J Cancer 2012;131:505-11. [Crossref] [PubMed]
- Kim H, An JY, Noh SH, et al. High microsatellite instability predicts good prognosis in intestinal-type gastric cancers. J Gastroenterol Hepatol 2011;26:585-92. [Crossref] [PubMed]
- Beghelli S, de Manzoni G, Barbi S, et al. Microsatellite instability in gastric cancer is associated with better prognosis in only stage II cancers. Surgery 2006;139:347-56. [Crossref] [PubMed]
- Mathiak M, Warneke VS, Behrens HM, et al. Clinicopathologic Characteristics of Microsatellite Instable Gastric Carcinomas Revisited: Urgent Need for Standardization. Appl Immunohistochem Mol Morphol 2015. [Epub ahead of print]. [Crossref] [PubMed]
- Bernal M, Ruiz-Cabello F, Concha A, et al. Implication of the β2-microglobulin gene in the generation of tumor escape phenotypes. Cancer Immunol Immunother 2012;61:1359-71. [Crossref] [PubMed]
- Wang L, Li W, Wang X, et al. Genetic alterations on chromosomes 3 and 9 of esophageal cancer tissues from China. Oncogene 1996;12:699-703. [PubMed]
- Kagawa Y, Yoshida K, Hirai T, et al. Microsatellite instability in squamous cell carcinomas and dysplasias of the esophagus. Anticancer Res 2000;20:213-7. [PubMed]
- Naidoo R, Ramburan A, Reddi A, et al. Aberrations in the mismatch repair genes and the clinical impact on oesophageal squamous carcinomas from a high incidence area in South Africa. J Clin Pathol 2005;58:281-4. [Crossref] [PubMed]
- Alexandrov LB, Nik-Zainal S, Wedge DC, et al. Signatures of mutational processes in human cancer. Nature 2013;500:415-21. [Crossref] [PubMed]
- Alexandrov LB, Nik-Zainal S, Wedge DC, et al. Deciphering signatures of mutational processes operative in human cancer. Cell Rep 2013;3:246-59. [Crossref] [PubMed]
- Snyder A, Makarov V, Merghoub T, et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N Engl J Med 2014;371:2189-99. [Crossref] [PubMed]
- Rizvi NA, Hellmann MD, Snyder A, et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science 2015;348:124-8. [Crossref] [PubMed]
- Peggs KS, Quezada SA, Chambers CA, et al. Blockade of CTLA-4 on both effector and regulatory T cell compartments contributes to the antitumor activity of anti-CTLA-4 antibodies. J Exp Med 2009;206:1717-25. [Crossref] [PubMed]
- Schneider H, Downey J, Smith A, et al. Reversal of the TCR stop signal by CTLA-4. Science 2006;313:1972-5. [Crossref] [PubMed]
- Postow MA, Chesney J, Pavlick AC, et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med 2015;372:2006-17. [Crossref] [PubMed]
- Gabrilovich DI, Ishida T, Nadaf S, et al. Antibodies to vascular endothelial growth factor enhance the efficacy of cancer immunotherapy by improving endogenous dendritic cell function. Clin Cancer Res 1999;5:2963-70. [PubMed]
- Escudier B, Bellmunt J, Négrier S, et al. Phase III trial of bevacizumab plus interferon alfa-2a in patients with metastatic renal cell carcinoma (AVOREN): final analysis of overall survival. J Clin Oncol 2010;28:2144-50. [Crossref] [PubMed]
- Pallasch CP, Leskov I, Braun CJ, et al. Sensitizing protective tumor microenvironments to antibody-mediated therapy. Cell 2014;156:590-602. [Crossref] [PubMed]
- Lake RA, Robinson BW. Immunotherapy and chemotherapy--a practical partnership. Nat Rev Cancer 2005;5:397-405. [Crossref] [PubMed]
- Zhang B, Bowerman NA, Salama JK, et al. Induced sensitization of tumor stroma leads to eradication of established cancer by T cells. J Exp Med 2007;204:49-55. [Crossref] [PubMed]
- Deng L, Liang H, Burnette B, et al. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J Clin Invest 2014;124:687-95. [Crossref] [PubMed]


