Advanced small cell lung cancer (SCLC): new challenges and new expectations
Introduction
Small cell lung cancer (SCLC) remains one of the most lethal malignancies and a major health riddle. Indeed the 5-year survival rate is less than 7% (1). Smoking is the main risk factor that is responsible for the high mutation burden of SCLC (2). The decrease of cigarette habit in Western societies has influenced the incidence of SCLC which has been diminished the last 20 years (3). The lack of specific symptoms at early tumor stages and the lack of screening methods remain the main obstacles for the early detection of the disease.
In contrast with non-small cell lung cancer (NSCLC), the origin of SCLC is unknown (4). Neuroendocrine cells (NECs) or neuroendocrine progenitors (NEPs) are the most possible progenitors of SCLC (1). On the other hand, the mixed type of SCLC and NSCLC may imply a common progenitor between these two entities (5). Ki67 is usually >50–70%, which indicates the high proliferation dynamic of this type of lung cancer; mitoses and necrosis are extensive (6). The tumor-suppressor genes TP53 and RB1 are mutated in the majority of patients with SCLC while PTEN mutations are present in 10–18% of SCLC (7,8).
The therapeutic options are limited. Lobectomy is recommended in stage I (T1–2N0M0) without mediastinal and supraclavicular disease (9). The combination of etoposide or irinotecan with platinum chemotherapy is the standard of care at any stage. In limited disease this schedule is combined with radiotherapy to thorax and mediastinum. In patients with complete response to first line treatment, prophylactic cerebral irradiation is indicated in order to prevent the progression of SCLC in brain. In extensive stage, chemotherapy without radiation is recommended. Unfortunately, the landscape in second line treatment is cloudy. Until now topotecan is the acceptance treatment but with modest results. Overall survival (OS) is 26 versus 14 weeks compared with best supportive care (10).
A better understanding of pathophysiologic mechanisms of initiation and progression of SCLC is necessary in order to develop more effective therapeutic options. The last decade systemic efforts have been done to reveal specific therapeutic targets for lung cancer. We hope that in next years the treatment of SCLC patients will be improved with the application of targeting therapy and the introduction of immunotherapy. In this review we focus on the new therapeutic strategies of SCLC, including immune-related treatment that may change the prognosis of the disease.
New challenges/new treatments
The low rates of PFS and OS in SCLC with the conventional treatments lead the scientists to the quest of other therapeutic approaches. Several well-known chemotherapeutic drugs including paclitaxel, docetaxel, gemcitabine, vinorelbine, temozolomide and ifosfamide has been studied in phase II clinical trials in second line therapy but the results are modest.
The most frequent genetic alterations observed in SCLC are TP53 and RB1 mutations; however, it is well known that these molecules cannot be a treatment target. In recent years, research has revealed other frequent genetic alterations and activated signaling pathways that might be an effective treatment target. Loss of PTEN, activating PI3K mutations, inhibition of NOTCH pathway and aurora kinase activation are some of them (8,11-14). Moreover, FDGFR1 amplification, activation of the Hedgehog pathway and repair-protein PARP1 seem to participate in tumorigenesis (7,15,16). These new findings have identified some interesting targets. Table 1 summarizes the most important investigated and probably promising drugs.
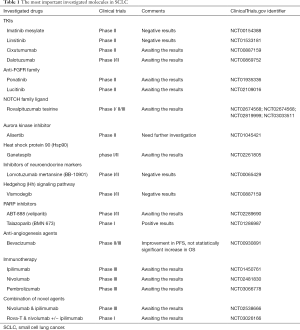
Full table
Rovalpituzumab tesirine (Rova-T, or S16LD6.5) is a humanized monoclonal antibody against the mammalian NOTCH family ligand delta-like 3 (DLL3) which is localized to the Golgi apparatus. DLL3 suppresses NOTCH signaling (17-19). In SCLC NOTCH signaling inhibits cancer development in contrast with other tumor types, therefore Rova-T enhances NOTCH signaling pathway (20,21). High expression of DLL3 has been observed in two thirds of patients with SCLC (19). Thus Rova-T in a phase Ia/Ib trial of patients with SCLC who received this agent as monotherapy after PD of first or second line chemotherapy showed efficacy and low toxicity in pretreated patients with DLL3 positive SCLC (19). Results of phase II clinical studies of Rova-T as first or second line therapy in patients with SCLC who express DLL3 are awaited. Moreover, the results of a phase III randomized, double blind placebo controlled trial of Rova-T as maintenance therapy following first line platinum based chemotherapy are ongoing.
PI3K/AKT/mTOR pathway is activated in great proportion in SCLC (22). Therefore, the molecules of this pathway are potentially targets of the treatment of SCLC. Indicatively, the most promising drugs: VS-5584, a selective dual inhibitor of mTORC1/2 and class I PI3-kinases (23) and BEZ235, another dual PI3K and mTORC1/2 inhibitor are investigating currently in early phase clinical trials. The key in the approach of SCLC with targeting the PI3K/AKT/mTOR pathway is the identification of predictive biomarkers.
Another interesting approach of the treatment of SCLC is the administration of aurora kinase inhibitor. Amplification of aurora A kinase lead the cell to proliferation, as it has been noticed in several cancer types. Moreover, aurora kinase A might have a predictive role in taxanes, as its amplification is associated with resistance to these drugs. Alisertib (MLN8237) is a reversible aurora kinase inhibitor that it is combined with paclitaxel in second line treatment and reveals an overall response rate of 21% in patients with advanced SCLC (24).
Several receptor tyrosine kinases inhibitors (TKIs) have been studied in clinical trials in patients with SCLC. Unfortunately, a lot of studies had negative results, such as the phase II trial of imatinib mesylate (STI571), an oral small molecule inhibitor of c-kit, which was studied as single agent or in combination with chemotherapy in previously untreated patients (25). Similarly, erlotinib, gefitinib, afatinib and linsitinib (OSI-906), an inhibitor of the IGF-1R tyrosine kinase did not show benefit in response rates of patients with relapsed SCLC (26). However, we are waiting the results of phase II clinical trial of cixutumumab and dalotuzumab (MK-0646), humanized monoclonal antibodies against IGF-I (27).
Another promising targeted therapy of SCLC is the anti-FGFR family, which is amplified in 5–6% of SCLC (28). Ponatinib, an FGFR inhibitor and BIBF1120, an FGFR, VEGFR and PDGFR inhibitor, are being investigated. Lucitanib another anti-FGFR1-3, anti-VEGFR1-3 and PDGFR α/β is studied in a recent phase II trial and the results are in progress. Further analysis of FGFR and FGFR ligand will help understanding better the behavior of SCLC and select the proper targeted treatment.
Targeting anti-apoptotic molecules in SCLC has not given any hoping result yet. The most well-known anti-apoptotic target is BCL-2, as it is overexpressed in SCLC (29). It has been observed in cell lines that BCL-2 is a potential a predictive marker for the combination of cisplatin with etoposide, as the inhibition of BCL-2 seems to increase the efficacy of this chemotherapeutic schedule (30). Several BCL-2 inhibitors have been studied till now, but despite the good results in preclinical trials and in phase I clinical trials, they give discouraging response rates in phase II trials (31-33).
Rinociclib is a CDK7 inhibitor that is evaluated in a phase Ib/II clinical trial in combination with chemotherapy as first line treatment in patients with extended disease SCLC (34). Histone deacetylase (HDAC) inhibitors induce apoptosis in cancer cells. Preclinical data gave hope to scientists, but the results of phase II clinical trial did not show effectiveness (35). Targeting heat shock protein 90 (Hsp90) in mouse xenograft models seems to inhibit the progression of SCLC. The efficacy and toxicity of ganetespib, an HSP90 inhibitor, in phase I and II clinical studies is awaited (36). Another interesting approach of SCLC is the inhibition of neuroendocrine markers (37). More precisely, lorvotuzumab mertansine (BB-10901), a humanized anti-CD56 monoclοnal antibody has already been used in xenograft models with promising results. A phase II clinical trial tried to evaluate the safety and efficacy of BB-10901 in patients with CD56 expressing tumors but the results were negative (38).
The finding of the Hedgehog (Hh) signaling pathway gave hope to the scientific society that its molecule cascade might be effective targets for the treatment of SCLC. Several preclinical trials have been done in order to inhibit Hh pathway, revealing a synergistic role between chemotherapy and Hh pathway (15). Subsequently, several inhibitors of Hh signaling pathway are being studying in clinical trials phase I or II (39). The most promising drug is vismodegib (GDC-0449), a small molecule that blocks Hh-ligand cell surface receptors PTCH and/or SMO and thus suppresses Hh signaling. Vismodegib has been evaluated in a phase II clinical trial with combination with cisplatin and etoposide in patients with extensive disease SCLC, but with no statistically benefit in PFS and OS compared with chemotherapy alone (40).
Poly ADP ribose polymerase 1 (PARP) inhibitors are also used in clinical trials of SCLC and a variety of predictive biomarkers are investigated. These clinical trials are based on the fact that PARP1 expression in SCLC is higher among all the subtypes of lung cancer (41). Moreover, it is found that PARP inhibition may enhance the sensitivity of platinum-based chemotherapy and radiation (40). A phase I trial (ECOG-ACRIN E2518) of patients with SCLC who received the PARP inhibitor ABT-888 (veliparib) in combination with cisplatin and etoposide had promising results and now phase 2 study is ongoing (42,43). Talazoparib (BMN 673), a newer PARP1/2 inhibitor is studied in preclinical models with promising results (44) and phase I study revealed some clinical benefit with acceptable toxicity.
Anti-angiogenesis agents and particularly the humanized monoclonal antibody targeting VEGF bevacizumab are used in clinical trials either as a second line treatment in combination with standard chemotherapy, or as a first line option. More precisely, in phase II clinical studies in patients with extensive SCLC bevacizumab seems to have some benefit (45-48). The results of phase III trial in patients with extensive SCLC who receive either bevacizumab with chemotherapy or chemotherapy alone in the first line therapy were disappointing. There was an improvement in PFS, but not a statistically significant increase in OS (49). Moreover, besides bevacizumab, other anti-angiogenetic agents such as sunitinib, sorafenib, vandetanib, pazopanib, aflibercept and thalidomide, have been studying in SCLC in phase I or II clinical trials with controversial results. These molecules have been studying as monotherapy in maintenance treatment after the standard schedule or in combination with chemotherapy, but they show none or minor clinical benefit and high toxicity (50-52).
The field of immunotherapy in several types of cancer has recently gives hope for cancer treatment. Immunotherapy tries to find its place in the treatment of SCLC (53). It has been observed increased T-cells infiltration in patients with limited SCLC that was reversed in those with advanced disease (54). Furthermore, increased PD-L1 expression was found in SCLC, underlying potential efficacy of the anti PD-1/PD-L1 agents. Studies in NSCLC and melanoma showed that immune checkpoint inhibitors are effective in the treatment of smoking related cancers with high somatic mutations (55,56).
Therefore, immune checkpoint inhibitors are under investigation in phase I and II clinical trials. More precisely, ipilimumab, an anti-CTL4 inhibitor, is studied in a phase II clinical trial in advanced SCLC showing improved PFS and OS compared to standard chemotherapy without the addition of immunotherapy (57). In an ongoing phase III trial, the addition of ipilimumab to standard etoposide-platinum chemotherapy in patients with extensive SCLC is being investigated. Anti PD-1 monoclonal antibodies, pembrolizumab and nivolumab, are also under study in several clinical trials. Two interesting phase III studies are ongoing: nivolumab versus topotecan as second line treatment of SCLC and pembrolizumab with etoposide/platinum in patients with extensive stage of SCLC. It is observed that PDL-1 expression is associated with longer survival in SCLC patients (58). However, it is uncertain if PDL-1 expression is a determinant predictive biomarker.
An interesting view of all the above novel agents is their combination. More precisely, the combination of ipilimumab and nivolumab or Rova-T plus nivolumab plus ipilimumab is under investigation. The efficacy of these schedules is unknown and even the toxicity is unclear. All the above studied molecules give hope in scientific committee that the physical progression of SCLC will be change soon.
Conclusions/expectations
SCLC is a fatal disease and a challenge for modern researchers. The exact pathogenesis is unclear and diagnostic, prognostic or predictive biomarkers are unknown. That makes the field of study even more difficult. This review is an effort to describe comprehensively the most important and promising therapeutic agents that could hopefully stop the mortal progression of the disease. The main clinical studies that showed effectiveness and might be a crucial step for the next level of clinical trials are reported.
The first line treatment, including etoposide in Western countries or irinotecan in Eastern countries with cisplatin or carboplatin, is unchangeable the last decades and no other known agent has managed to give better response rates. However, all patient present local recurrence or distant metastasis thus the disease is uncontrolled. As far as the second line treatment is concern there is a cloudy field.
Now it is known that SCLC is not one entity as it was thought before, but there is heterogeneity in patients. There is a necessity to continue basic research and to develop more clinical trials for better understand and control this disease. We should be able to distinguish the groups of patients that will be benefit for specific treatments. For example, patients with limited disease might have different mutational burden in comparison with patients in extended disease and scientists should find these trigger mutations in order to develop new effective drugs (59).
New drugs and combinations of a variety of novel agents such as aurora kinase inhibitors, anti-apoptotic agents and PARP inhibitors are investigating in phase I or II clinical trials but the results are controversial. Unfortunately, the majority of trials did not fulfill the expectations. Immunotherapy seems to change the progression of the disease but there is needed time to reveal its exact role in the treatment of SCLC. Scientists must take into account all the available genetic information and the results from ex vivo and in vivo studies in order to perceive the clinically effective information. Bioinformatics is a useful tool that we have to take advantage. Liquid biopsies, next generation sequencing technics and the tumor genotyping not only in baseline but also in advanced stages will help us find predictive biomarkers and new more effective drugs. There is hope that the next years the approach of patients with SCLC will change and their OS will be increased.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Karachaliou N, Pilotto S, Lazzari C, et al. Cellular and molecular biology of small cell lung cancer: an overview. Transl Lung Cancer Res 2016;5:2-15. [PubMed]
- Pleasance ED, Stephens PJ, O'Meara S, et al. A small-cell lung cancer genome with complex signatures of tobacco exposure. Nature 2010;463:184-90. [Crossref] [PubMed]
- Govindan R, Page N, Morgensztern D, et al. Changing epidemiology of small-cell lung cancer in the United States over the last 30 years: analysis of the surveillance, epidemiologic, and end results database. J Clin Oncol 2006;24:4539-44. [Crossref] [PubMed]
- Sutherland KD, Proost N, Brouns I, et al. Cell of origin of small cell lung cancer: inactivation of Trp53 and Rb1 in distinct cell types of adult mouse lung. Cancer Cell 2011;19:754-64. [Crossref] [PubMed]
- Yesner R. Heterogeneity of so-called neuroendocrine lung tumors. Exp Mol Pathol 2001;70:179-82. [Crossref] [PubMed]
- Nicholson SA, Beasley MB, Brambilla E, et al. Small cell lung carcinoma (SCLC): a clinicopathologic study of 100 cases with surgical specimens. Am J Surg Pathol 2002;26:1184-97. [Crossref] [PubMed]
- Peifer M, Fernández-Cuesta L, Sos ML, et al. Integrative genome analyses identify key somatic driver mutations of small-cell lung cancer. Nat Genet 2012;44:1104-10. [Crossref] [PubMed]
- Yokomizo A, Tindall DJ, Drabkin H, et al. PTEN/ MMAC1 mutations identified in small cell, but not in nonsmall cell lung cancers. Oncogene 1998;17:475-9. [Crossref] [PubMed]
- Schneider BJ, Saxena A, Downey RJ. Surgery for early stage small cell lung cancer. J Natl Compr Canc Netw 2011;9:1132-9. [Crossref] [PubMed]
- O’Brien ME, Ciuleanu TE, Tsekov H, et al. Phase III trial comparing supportive care alone with supportive care with oral topotecan in patients with relapsed small-cell lung cancer. J Clin Oncol 2006;24:5441-7. [Crossref] [PubMed]
- Sarvesvaran J, Going JJ, Milroy R, et al. Is small cell lung cancer the perfect target for anti-telomerase treatment? Carcinogenesis 1999;20:1649-51. [Crossref] [PubMed]
- Shibata T, Kokubu A, Tsuta K, et al. Oncogenic mutation of PIK3CA in small cell lung carcinoma: a potential therapeutic target pathway for chemotherapy-resistant lung cancer. Cancer Lett 2009;283:203-11. [Crossref] [PubMed]
- Noguchi M, Hirohashi S, Hara F, et al. Heterogenous amplification of myc family oncogenes in small cell lung carcinoma. Cancer 1990;66:2053-8. [Crossref] [PubMed]
- Tamborini E, Bonadiman L, Negri T, et al. Detection of overexpressed and phosphorylated wild-type kit receptor in surgical specimens of small cell lung cancer. Clin Cancer Res 2004;10:8214-9. [Crossref] [PubMed]
- Park KS, Martelotto LG, Peifer M, et al. A crucial requirement for Hedgehog signaling in small cell lung cancer. Nat Med 2011;17:1504-8. [Crossref] [PubMed]
- Byers LA, Wang J, Nilsson MB, et al. Proteomic profiling identifies dysregulated pathways in small cell lung cancer and novel therapeutic targets including PARP1. Cancer Discov 2012;2:798-811. [Crossref] [PubMed]
- Chapman G, Sparrow DB, Kremmer E, et al. Notch inhibition by the ligand DELTA-LIKE 3 defines the mechanism of abnormal vertebral segmentation in spondylocostaldysostosis. Hum Mol Genet 2011;20:905-16. [Crossref] [PubMed]
- Geffers I, Serth K, Chapman G, et al. Divergent functions and distinct localization of the Notch ligands DLL1 and DLL3 in vivo. J Cell Biol 2007;178:465-76. [Crossref] [PubMed]
- Pietanza MC, Spigel D, Bauer TM, et al. Safety, activity, and response durability assessment of single agent rovalpituzumabtesirine, a delta-like protein3 (DLL3)- targeted anibody drug conjugate (ADC), in small cell lung cancer (SCLC) [abstract LBA 7]. European Cancer Congress, Vienna, Austria, 2015.
- Espinoza I, Miele L. Notch inhibitors for cancer treatment. Pharmacol Ther 2013;139:95-110. [Crossref] [PubMed]
- Kunnimalaiyaan M, Chen H. Tumor suppressor role of Notch-1 signaling in neuroendocrine tumors. Oncologist 2007;12:535-42. [Crossref] [PubMed]
- Umemura S, Mimaki S, Makinoshima H, et al. Therapeutic priority of the PI3K/AKT/mTOR pathway in small cell lung cancers as revealed by a comprehensive genomic analysis. J Thorac Oncol 2014;9:1324-31. [Crossref] [PubMed]
- Kolev VN, Xu Q, Padval M, et al. FAK and PI3K/mTOR Inhibitors Target Cancer Stem Cells: Implications for SCLC Treatment Strategies. Cancer Res 2015;75:1525. [Crossref]
- Melichar B, Adenis A, Lockhart AC, et al. Safety and activity of alisertib, an investigational aurora kinase A inhibitor, in patients with breast cancer, small-cell lung cancer, non-small-cell lung cancer, head and neck squamous-cell carcinoma, and gastro- esophageal adenocarcinoma: a five-arm phase 2 study. Lancet Oncol 2015;16:395-405. [Crossref] [PubMed]
- Decaudin D, de Cremoux P, Sastre X, et al. In vivo efficacy of STI571 in xenografted human small cell lung cancer alone or combined with chemotherapy. Int J Cancer 2005;113:849-56. [Crossref] [PubMed]
- Riely GJ, Yu HA. EGFR: The Paradigm of an Oncogene- Driven Lung Cancer. Clin Cancer Res 2015;21:2221-6. [Crossref] [PubMed]
- Arcaro A. Targeted therapies for small cell lung cancer: Where do we stand? Crit Rev Oncol Hematol 2015;95:154-64. [Crossref] [PubMed]
- Schultheis AM, Bos M, Schmitz K, et al. Fibroblast growth factor receptor 1 (FGFR1) amplification is a potential therapeutic target in small-cell lung cancer. Mod Pathol 2014;27:214-21. [Crossref] [PubMed]
- Kaiser U, Schilli M, Haag U, et al. Expression of bcl- 2-protein in small cell lung cancer. Lung Cancer 1996;15:31-40. [Crossref] [PubMed]
- Zangemeister-Wittke U, Schenker T, Luedke GH, et al. Synergistic cytotoxicity of bcl-2 antisense oligodeoxynucleotides and etoposide, doxorubicin and cisplatin on small-cell lung cancer cell lines. Br J Cancer 1998;78:1035-42. [Crossref] [PubMed]
- Rudin CM, Salgia R, Wang X, et al. Randomized phase II study of carboplatin and etoposide with or without the bcl-2 antisense oligonucleotide oblimersen for extensive stage small-cell lung cancer: CALGB 30103. J Clin Oncol 2008;26:870-6. [Crossref] [PubMed]
- Paik PK, Rudin CM, Pietanza MC, et al. A phase II study of obatoclaxmesy late, a Bcl-2 antagonist, plus topotecan in relapsed small cell lung cancer. Lung Cancer 2011;74:481-5. [Crossref] [PubMed]
- Rudin CM, Hann CL, Garon EB, et al. Phase II study of single-agent navitoclax (ABT-263) and biomarker correlates in patients with relapsed small cell lung cancer. Clin Cancer Res 2012;18:3163-9. [Crossref] [PubMed]
- Christensen CL, Kwiatkowski N, Abraham BJ, et al. Targeting transcriptional addictions in small cell lung cancer with a covalent CDK7 inhibitor. Cancer Cell 2014;26:909-22. [Crossref] [PubMed]
- de Marinis F, Atmaca A, Tiseo M, et al. A phase II study of the histone deacetylase inhibitor panobinostat (LBH589) in pretreated patients with small-cell lung cancer. J Thorac Oncol 2013;8:1091-4. [Crossref] [PubMed]
- Lai CH, Park KS, Lee DH, et al. HSP-90 inhibitor ganetespib is synergistic with doxorubicin in small cell lung cancer. Oncogene 2014;33:4867-76. [Crossref] [PubMed]
- Semenova EA, Nagel R, Berns A. Origins, genetic landscape, and emerging therapies of small cell lung cancer. Genes Dev 2015;29:1447-62. [Crossref] [PubMed]
- Socinski MA, Kaye FJ, Spigel DR, et al. Phase 1/2 Study of the CD56-Targeting Antibody-Drug Conjugate Lorvotuzumab Mertansine (IMGN901) in Combination With Carboplatin/Etoposide in Small-Cell Lung Cancer Patients With Extensive-Stage Disease. Clin Lung Cancer 2017;18:68-76.e2. [Crossref] [PubMed]
- Maulik G, Kijima T, Ma PC, et al. Modulation of the c-Met/hepatocyte growth factor pathway in small cell lung cancer. Clin Cancer Res 2002;8:620-7. [PubMed]
- Belani CP, Dahlberg SE, Rudin CM, et al. Three-arm randomized phase II study of cisplatin and etoposide (CE) versus CE with either vismodegib (V) or cixutumumab (Cx) for patients with extensive stage-small cell lung cancer (ES-SCLC) (ECOG 1508). J Clin Oncol 2013;31:abstr7508.
- Santarpia M, Grazia-Daffina M, Karachaliou N, et al. Targeted drugs in small-cell lung cancer. Transl Lung Cancer Res 2016;5:51-70. [PubMed]
- Owonikoko TK, Zhang G, Deng X, et al. Poly (ADP) ribose polymerase enzyme inhibitor, veliparib, potentiates chemotherapy and radiation in vitro and in vivo in small cell lung cancer. Cancer Med 2014;3:1579-94. [Crossref] [PubMed]
- Owonikoko TK, Dahlberg SE, Khan SA, et al. A phase 1 safety study of veliparib combined with cisplatin and etoposide in extensive stage small cell lung cancer: A trial of the ECOG-ACRIN Cancer Research Group (E2511). Lung Cancer 2015;89:66-70. [Crossref] [PubMed]
- Cardnell RJ, Feng Y, Diao L, et al. Proteomic markers of DNA repair and PI3K pathway activation predict response to the PARP inhibitor BMN 673 in small cell lung cancer. Clin Cancer Res 2013;19:6322-8. [Crossref] [PubMed]
- Ready NE, Dudek AZ, Pang HH, et al. Cisplatin, irinotecan, and bevacizumab for untreated extensive-stage small-cell lung cancer: CALGB 30306, a phase II study. J Clin Oncol 2011;29:4436-41. [Crossref] [PubMed]
- Spigel DR, Greco FA, Zubkus JD, et al. Phase II trial of irinotecan, carboplatin, and bevacizumab in the treatment of patients with extensive-stage small-cell lung cancer. J Thorac Oncol 2009;4:1555-60. [Crossref] [PubMed]
- Horn L, Dahlberg SE, Sandler AB, et al. Phase II study of cisplatin plus etoposide and bevacizumab for previously untreated, extensive-stage small-cell lung cancer: Eastern Cooperative Oncology Group Study E3501. J Clin Oncol 2009;27:6006-11. [Crossref] [PubMed]
- Spigel DR, Townley PM, Waterhouse DM, et al. Randomized phase II study of bevacizumab in combination with chemotherapy in previously untreated extensive-stage small-cell lung cancer: results from the SALUTE trial. J Clin Oncol 2011;29:2215-22. [Crossref] [PubMed]
- Tiseo M, Boni L, Ambrosio F, et al. Italian multicenter phase III randomized study of cisplatin-etoposide with or without bevacizumab as first-line treatment in extensive stage small cell lung cancer: treatment rationale and protocol design of the GOIRC-AIFA FARM6PMFJM trial. Clin Lung Cancer 2015;16:67-70. [Crossref] [PubMed]
- Sharma N, Pennell N, Nickolich M, et al. Phase II trial of sorafenib in conjunction with chemotherapy and as maintenance therapy in extensive-stage small cell lung cancer. Invest New Drugs 2014;32:362-8. [Crossref] [PubMed]
- Ready NE, Pang HH, Gu L, et al. Chemotherapy With or Without Maintenance Sunitinib for Untreated Extensive- Stage Small-Cell Lung Cancer: A Randomized, Double- Blind, Placebo Controlled Phase II Study-CALGB 30504 (Alliance). J Clin Oncol 2015;33:1660-5. [Crossref] [PubMed]
- Allen JW, Moon J, Redman M, et al. Southwest Oncology Group S0802: a randomized, phase II trial of weekly topotecan with and without ziv-aflibercept in patients with platinum-treated small-cell lung cancer. J Clin Oncol 2014;32:2463-70. [Crossref] [PubMed]
- Sharma P, Allison JP. The future of immune checkpoint therapy. Science 2015;348:56-61. [Crossref] [PubMed]
- Koyama K, Kagamu H, Miura S, et al. Reciprocal CD4+ T-cell balance of effector CD62Llow CD4+ and CD62LhighCD25+ CD4+ regulatory T cells in small cell lung cancer reflects disease stage. Clin Cancer Res 2008;14:6770-9. [Crossref] [PubMed]
- Snyder A, Makarov V, Merghoub T, et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N Engl J Med 2014;371:2189-99. [Crossref] [PubMed]
- Hellmann MD, Creelan BC, Woo K, et al. 1229PD: Smoking history and response to nivolumab in patients with advanced NSCLCS. Ann Oncol 2014;25:iv429. [Crossref]
- Reck M, Bondarenko I, Luft A, et al. Ipilimumab in combination with paclitaxel and carboplatin as first-line therapy in extensive-disease-small-cell lung cancer: results from a randomized, double-blind, multicenter phase 2 trial. Ann Oncol 2013;24:75-83. [Crossref] [PubMed]
- Ishii H, Azuma K, Kawahara A, et al. Significance of programmed cell death-ligand 1 expression and its association with survival in patients with small cell lung cancer. J Thorac Oncol 2015;10:426-30. [Crossref] [PubMed]
- Koinis F, Kotsakis A, Georgoulias V. Small cell lung cancer (SCLC): no treatment advances in recent years. Transl Lung Cancer Res 2016;5:39-50. [PubMed]


