Abstract
Long noncoding RNAs are emerging as key players in various fundamental biological processes. We highlight the varied molecular mechanisms by which lncRNAs modulate gene expression in diverse cellular contexts and their role in early mammalian development in this review. Furthermore, it is being increasingly recognized that altered expression of lncRNAs is specifically associated with tumorigenesis, tumor progression and metastasis. We discuss various lncRNAs implicated in different cancer types with a focus on their clinical applications as potential biomarkers and therapeutic targets in the pathology of diverse cancers.
Similar content being viewed by others
Introduction
The ‘Central Dogma’ of life describes the flow of genetic information from DNA to proteins involving RNA as an intermediate. The view of DNA being the store house of genetic information and proteins as its functional manifestation dominated the field of biology for several decades. However, the proposal of the famous RNA world hypothesis marked the beginning of an era where RNA was attributed more recognition in terms of cellular and physiological functionality rather than being considered only as a messenger between DNA and proteins [1]. After the completion of Human Genome Project it became evident that only a small portion of the genome encodes proteins [2, 3]. Further, advancements in tiling array and high throughput analyses revealed that the mammalian genome is pervasively transcribed [4, 5] and it was speculated that the large number of noncoding RNAs may reflect transcriptional noise. However, recent developments in the field of RNA biology have consolidated the fact that noncoding RNAs (ncRNAs) are indeed crucial molecules playing diverse regulatory roles in development and disease. On the basis of their main biological functions, ncRNAs are broadly classified as structural and regulatory ncRNAs. Structural ncRNAs have been known since a long time because of their role as essential components of the protein synthesis machinery [6]. These include transfer RNA (tRNA), ribosomal RNA (rRNA), small nuclear RNA (snRNA) and small nucleolar RNA (snoRNA). Regulatory ncRNAs include small interfering RNA (siRNA), microRNA (miRNA), piwi-RNA (piRNA) and long noncoding RNA (lncRNA) [7–9]. LncRNAs are arbitrarily defined as non coding transcripts of more than 200 nt in length. Most lncRNAs annotated till date have been reported to lack protein coding capacity, albeit few of them have the capacity to code for small peptides that had not been identified previously [10]. Based on their genomic location, lncRNAs are classified as sense lncRNA (lncRNA sequence overlapping with the sense strand of a protein coding gene), antisense lncRNA (lncRNA sequence overlapping with the antisense strand of protein coding gene), bidirectional (lncRNA sequence located on the opposite strand of a protein coding gene), intronic (lncRNA derived from an intron of a gene) and intergenic (lncRNA derived from region between two genes). In the present review we focus on the general features of lncRNA, their mechanisms of action and their role in development and cancer.
Similarities and differences between lncRNA and mRNA
Irrespective of the major differences between mRNA and lncRNA with reference to their protein coding capacity, they share some common features as well. Similar to mRNA, most lncRNAs are transcribed by RNAPolII machinery and actively transcribed lncRNA genes possess histone modification signatures similar to that of protein coding genes [11, 12]. The ‘K4-K36 domain’ that refers to the distinctive chromatin signature of H3K4me3 modification at the promoters and H3K36me3 modification along the gene body of RNAPolII transcribed genes is present on a host of non-protein coding multi-exonic transcripts [11]. Furthermore, majority of lncRNAs are also polyadenylated and the pathway of biogenesis of lncRNA and mRNA cannot be distinguished from each other [13]. Studies have also revealed similarities between lncRNA and the 3’UTR region of mRNA mainly with respect to their secondary structures, sequence composition and thermodynamic parameters [14, 15]. Sequence conservation is a feature that distinguishes lncRNA and protein coding RNA. Many studies including recent lncRNA datasets identified from different species have shown the poor conservation of lncRNA sequences across species as compared to protein coding genes [16–18]. However, within their sequence, many lncRNAs have regions which exhibit very high conservation suggesting that key functional domains may be the ones that retain their identity over the evolutionary time period.
Regulation of gene expression by lncRNAs
It is being increasingly recognized that lncRNAs play a critical role in modulating genetic networks and signal transduction pathways during development and their deregulation leads to disease phenotypes [19, 20]. Several molecular mechanisms have been delineated for lncRNA mediated regulation of gene expression [21]. These molecular mechanisms include a) LncRNAs acting as decoys by binding to transcription factors and preventing the binding of these factors to their regulatory DNA elements [22]; b) Formation of triple helix with target DNA sequences [23]; c) LncRNAs titrating out miRNAs from their regulatory mRNA targets by binding to the specific miRNAs (miRNA sponge mechanism) [24]; d) LncRNAs as scaffold, which is one of the most common mechanisms employed by diverse lncRNAs [25, 26], e) LncRNAs acting as tethers to recruit protein partners resulting in the formation of functional ribonucleoprotein complexes [27]; f) Modulation of mRNA translation [28]; g) Modulation of splicing [29] and h) mRNA degradation [30]. Further, lncRNAs can serve as precursors for small RNAs like piRNAs, miRNAs or snoRNAs which can further perform their regulatory functions [31–33]. Other than their regulatory role in gene expression, lncRNAs also contribute to the organization of different nuclear structures [34, 35]. These mechanisms are pictorially depicted in Fig. 1. Besides, lncRNAs broadly regulate gene expression at epigenetic, transcriptional, post transcriptional levels and by cell-cell signaling through hormones as discussed below.
Diverse mechanisms of lncRNA function. Various studies have elucidated different mechanisms of function by lncRNA. One example of lncRNA for each mechanism is mentioned in the bracket. a) LncRNAs can function as decoys by binding to a transcription factor and preventing its action on the target DNA. b) LncRNAs modulate gene expression by recruiting chromatin modifiers. c) LncRNAs regulate various biological processes by being a part of RNP component, regulating the activity or localization of a particular protein and playing a structural role in organization within the nucleus. d) LncRNAs act as miRNA sponges by titrating the miRNAs away from their mRNA targets. e) LncRNAs modulate the translation and degradation of their mRNA targets. f) LncRNA can modulate the splicing of pre-mRNA. lncRNA, long non coding RNA; mRNA, messenger RNA; RNP, Ribonucleoprotein
Epigenetic mode of gene regulation by lncRNAs
A large number of lncRNAs remain in the nucleus and play an essential role in shaping the epigenome either by genomic imprinting or through chromatin remodeling as described below.
By genomic imprinting
Genomic Imprinting refers to the phenomenon of epigenetic silencing of an allele inherited from either of the parents [36]. Short stretches of DNA known as Imprinting Control Regions (IRCs) play a critical role in imprinting of multiple genes [37]. Interestingly, it has been observed that the imprinted regions show significant association with ncRNAs, which mediate the silencing by diverse mechanisms like chromatin remodeling and enhancer competition [38].
Through chromatin modifying complexes
The principal means by which most of the lncRNAs regulate gene expression is by recruiting chromatin remodelers to facilitate histone modifications at specific gene loci either for the repression or activation of the target genes [39]. Various lncRNAs have been shown to employ chromatin modifying complexes like Polycomb Repressive Complexes 1 and 2 (PRC1 and PRC2) [40–43], CoREST (CoRepressor for element-1-silencing transcription factor) [44], SMCX (Smcy homolog, X-linked), [45], G9a [46], LSD1 (Lysine Specific Demethylase 1) [47], Trithorax (Trx) activating complex [48], etc. to regulate gene expression, as discussed in detail later in this review.
Transcriptional regulation of gene expression by lncRNAs
Recent studies have elucidated the fact that several lncRNAs modulate gene expression by specifically associating either at the promoters or the enhancers of their target genes.
Promoter-associated lncRNAs (plncRNAs/ pRNAs)
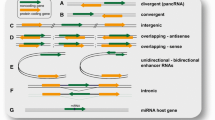
Divergent transcription at the promoter regions of various genes gives rise to lncRNAs which in turn regulate the transcription of the neighboring genes [49–52]. For example, Hung et al. [53] studied the chromatin landscape around transcription start site (TSS) of 56 cell cycle associated genes and showed that 49 of these genes are associated with at least one lncRNA. One of these, PANDA (Promoter of CDKN1A Antisense DNA Damage Activated RNA), produced from the CDKN1A promoter region, shows p53 dependent induction after DNA damage and aids in cell proliferation by inhibiting the apoptotic genes [53]. Further, Negishi et al. [54] reported a novel lncRNA, APTR (Alu-mediated p21Transcriptional Regulator) that represses the transcription of CDK inhibitor p21 by recruiting PRC2 complex to the p21 promoter. LincRNA-p21 is another promoter associated lncRNA that acts as a p53 dependent tumor suppressor. It gets localized to promoters of genes that are repressed by p53, facilitating their inhibition through hnRNP-K [55, 56].
Enhancer-associated lncRNAs (elncRNAs/ eRNAs)
Enhancers are critical regulatory elements required for the tight developmental and tissue specific regulation of gene expression and hence it is not surprising that the number of enhancers in mammalian genome far exceeds the number of protein coding genes [57, 58]. Very recently, several studies have revealed that enhancers give rise to lncRNAs that are suggested to be crucial for mediating gene regulation, both positively and negatively, by mediating chromosomal looping [59–63]. Additionally, super enhancers have been reported which consist of clusters of enhancers, mainly associated with genes involved in maintenance of cell identity [64, 65]. Super enhancers are also known to be associated with lncRNAs. For example the lncRNA, CCAT-1 L (Colorectal Cancer Associated Transcript 1-Long isoform) transcribed from an upstream super enhancer locus of the oncogene, Myc (Myelocytomatosis) functions as an eRNA and plays a role in transcription regulation of Myc [66].
Post transcriptional regulation
LncRNAs are also widely implicated in the post transcriptional regulation of mRNAs including splicing, transport, translation and degradation. For example, MALAT1 is involved in splicing events [67], discussed in detail later. Certain other lncRNAs have been implicated in stabilizing and promoting the translation of mRNAs by extended base pairing with them [68]. In addition, lncRNAs can also facilitate the inhibition of mRNA translation or decay by partial base pairing with the 3’UTR sequences through their Alu elelemts in Staufen-mediated manner [30].
Regulation through hormone responsive genes
Some lncRNAs regulate gene expression through their interaction with hormone receptors. For example, SRA (Steroid receptor RNA Activator) lncRNA is a coactivator of various steroid hormone receptors like GR (Glucocorticoid Receptor), AR (Androgen Receptor), ER (Estrogen Receptor) and PR (Progesterone Receptor) [69]. GAS5 is another lncRNA that participates in hormone mediated gene regulation [22].
LncRNAs in early mammalian development
Widespread studies have established that lncRNAs participate in a variety of mammalian developmental processes like regulating lineage commitment and cell fate decisions, in organogenesis, in imprinting of alleles during early development and also in specification of the body pattern. A majority of the lncRNAs exhibit a tissue-specific expression pattern [12], which helps in fine tuning and coordinating the context-dependent signals to regulate the cellular physiology when compared to the more ubiquitously expressed protein molecules. Interestingly, many of the lncRNAs involved in regulating development contribute to various disease pathologies including cancer, when altered [70, 71]. Recent studies have identified cancer stem cells as the main players that drive cancer progression in most cases. Cancer stem cells bear striking similarities with the on setters of development i.e., embryonic stem cells. Both these type of cells possess unlimited proliferative capacity and harbor the potential to migrate to specific destinations by undergoing epithelial to mesenchymal transition. Under such circumstances, it would be worthwhile to study how the multitude of lncRNAs that govern developmental cues can also lead to various disorders, developmental in nature or otherwise.
Dosage compensation
An excellent example of genome level regulation has been provided by the discovery of XIST (X inactive-specific transcript). Being involved in inactivating one of the pair of X chromosomes, its expression is restricted mainly to females and further only from the X-chromosome that will be inactivated in the future [72]. Analysis of the conservation pattern between mouse and human XIST reveals identical stretches of sequences with interspersed non-conserved regions, suggesting that over evolution, principle functional domains have been retained. The locus has been shown to transcribe a couple of lncRNAs including REPA and TSIX. While REPA is derived from XIST and acts to recruit the PRC2 complex to inactivate the future Xi (inactive X chromosome) via H3K27 trimethylation of the chromatin [73], TSIX is the antisense repressor of XIST and prevents inactivation of the future Xa (active X-chromosome) [74]. Like the negative regulator of XIST, there exists a positive regulator, JPX, that in turn is produced from the X-inactivation center and exerts its action in trans to activate XIST on Xi [75]. This TSIX-JPX switch for Xa-Xi provides a wonderful illustration of RNA-based transcriptional control.
Loss of function studies for XIST further emphasize its importance in mammalian development. Marahrens et al. [76] generated a targeted partial deletion of the Xist gene and interestingly discovered that mutant males were unaffected by the deletion along with mutant females who inherited the deleted gene maternally. However, mutant females containing the paternally inherited deletion showed death early during embryogenesis. This was attributed to the expression of both X chromosomes in the extra-embryonic tissues that led to abnormalities in the development of the embryo proper.
Patterning of the body axes
The specification of the anterior-posterior body axis and determination of the positional identity of individual cells as well as organs is governed by a group of homeodomain containing proteins, encoded by the Hox clusters of genes. LncRNAs have been associated with this phenomenon, a predominant one being HOTAIR [77]. It represents a classical example of the trans mode of action of lncRNAs as it is expressed from the HoxC locus in mammals but exerts its action at the HoxD locus. HOTAIR recruits the PRC2 complex at the target locus resulting in spreading of H3K27 trimethylation over the region and additionally interacts with the LSD1/REST/co-REST complex to perform lysine 4 demethylation, exemplifying the functioning of lncRNAs as molecular ‘scaffolds’ [25]. The Hox locus is in fact quite a storehouse of lncRNAs. HOTTIP is expressed at the 5’ end of the HoxA locus and recruits the WDR5/MLL complex across the locus by chromosomal looping, bringing about H3K4 trimethylation and subsequent gene transcription. Interestingly, its strength of action on the Hox genes decreases with increasing distance from its own site of transcription [78]. While HOTTIP has a more distal pattern of expression, another lncRNA at the HoxC locus, FRIGIDAIR has a function in anterior patterning [21]. The complex interplay between proteins and lncRNAs at such gene loci at the Hox loci is thus crucial in proper embryonic development.
Targeted deletion at the Hotair locus has revealed that the lncRNA is as essential as the HOX proteins for the proper development of the embryo [79]. Its absence leads to malformation of the skeletal system, massive derepression at several loci including that of HoxD and certain imprinted loci like Dlk1, Igf2 (paternally imprinted) and H19, Meg3 (maternally imprinted) amongst others. Perturbations in these genes further alters gene expression pattern in vivo leading to abnormalities during development.
Genomic imprinting
LncRNAs have also been implicated in genomic imprinting of specific alleles, a phenomenon that is a part of the early developmental regime. AIR (Antisense Igf2r RNA) is expressed in an antisense direction from the Igf2r (Insulin-like growth factor type2 receptor) locus, is maternally imprinted and assists in the imprinting of certain paternal genes like Slc22a2 and Slc22a3, expressed upstream from Air [80]. Early during embryonic development, in the placenta, AIR acts at the Slc22a3 promoter but not at the Igf2r promoter, by interacting with H3K9 methyl transferase, G9a [81]. KCNQ1OT1 is another example of a lncRNA participating in allelic imprinting. Being maternally imprinted and paternally expressed antisense to the Kcnq1 locus, it is involved in gene repression at various loci in the paternal genome that have been classified as ubiquitously imprinted (Kcnq1, Cdkn1c, Phlda2 and Slc22a18) or placental-specific imprinted (Osbpl5, Tssc4 and Ascl2) [82]. The lncRNA interacts with both G9a and PRC2 components to bring about imprinting as early as 3.5 to 5.5 dpc of embryonic development thereby playing an important role in specifying parental-specific gene expression [83].
LncRNA H19 is also involved in allelic imprinting, being expressed from the Igf2 locus and itself being paternally imprinted [84, 85]. It is highly expressed from the maternal allele during the blastocyst stage and later in endodermal and mesodermal tissues, but is restricted in expression only to skeletal tissues in the adults [84]. Knockout of the H19 gene results in mutant animals that are viable and fertile, showing an overweight phenotype probably due to a gain of biallelic expression of the previously imprinted Igf2 locus [85]. At the IGN (Imprinted Gene Network) locus, H19 acts to repress several genes including Igf2, Slc38a4 and Peg-1 by interacting with the methyl-CpG-binding domain protein, MBD1 [86]. The recruitment of this mediator protein to the IGN loci directs imprinting by bringing additional histone methyltransferases that drive repression of gene expression. Further, H19 acts as a precursor for the microRNA miR-675 that regulates placental growth [86].
LncRNAs have been well characterized in many cellular contexts and shown to help in maintenance of pluripotency of stem cells, in adult progenitor cell proliferation as well as in the differentiation of specific tissues [87–93]. Furthermore, their involvement in early mammalian development and in human diseases like cancer underlines their importance as an integral component of the pathways that regulate diverse physiological processes [94]. In view of this, the role of lncRNAs in cancer would be dealt with in detail in the upcoming section with special focus on their clinical and therapeutic relevance.
LncRNAs in cancer
Cancer arises due to accumulation of genetic and epigenetic alterations in cells. Gain or loss of chromosomes has also been frequently observed in cancer cells. Several signal transduction pathways like Wnt/ β Catenin, MAPK, TGFβ, p14ARF/p53, PI3K/ AKT etc. are altered in the malignant cells which seem to produce their own growth factors and attain replicative immortality, enhanced angiogenesis and proliferation. Further, they evade growth repressors, escape apoptosis and acquire the ability of metastasis and invasion [95]. Transcriptome profiling of tumor cells has elucidated a central role for the vast noncoding landscape of the human genome in tumorigenesis. Specifically, long noncoding RNAs are emerging as key players in genetics and pathogenesis of cancer and their dysfunction is closely associated with cancer development, progression and metastasis; reviewed in [96–104]. While some lncRNAs are oncogenic by nature and drive cancer conditions when up-regulated, some others act as tumor suppressors and cause cancer only when they are down-regulated [105]. Some of the important lncRNAs deregulated in cancer, their mechanism of action and their potential clinical applications are discussed below.
H19 is among the earliest lncRNAs discovered and identified to be key factor regulating gene expression [106]. Expression of H19 is developmentally orchestrated and in turn it determines the repression of multiple genes through genomic imprinting [107, 108]. Interestingly, this lncRNA itself is produced from a paternally imprinted, maternally expressed gene at 11p15.5 locus, adjacent to the oppositely imprinted IGF2 (Insulin like Growth Factor2) gene. It produces a 2.3 kb spliced, capped and polyadenylated lncRNA conserved between rodents and human and also is processed to an miRNA, miRNA-675 [109]. A recent study by Monnier et al. [86] has shown that H19 silences the genes in the Imprinted Gene Network (IGN) through MBD1 (Methyl CpG-binding domain protein1), which is responsible for the repressive histone mark H3K9me3. Though the knockout of H19 is not lethal in mice [110], its over-expression, either due to loss of imprinting (LOI) at H19 locus or due to the loss of tumor suppressor gene p53 [111], or under the influence of the oncogene, Myc [112], leads to the activation of genes involved in angiogenesis, cell survival and proliferation [113, 114], triggering several malignancies like liver [115, 116], breast [117], colorectal [118], esophageal [119], lung [120], pancreatic [121], gastric [122], bladder [123] and cervical [124] carcinomas suggesting an oncogenic function for this RNA. In fact level of H19 expression shows significant correlation with tumor grade and is a potential biomarker for various cancers [114, 123, 125, 126]. In contrast, miR-675, the miRNA derived from H19, exhibits antagonistic behavior and functions as a tumor suppressor by repressing the IGF1R (Insulin like Growth Factor 1 Receptor) expression [127], thus the levels of these two transcripts help in maintaining cellular homeostasis.
KCNQ1OT1 (KCNQ1 Overlapping Transcript 1) is another imprinted, paternally expressed 91.5 kb transcript produced from the KCNQ1 locus, a few hundred kilobases away from H19 [128]. It regulates gene expression epigenetically by interacting with chromatin remodeling complexes like PRC1, PRC2 and G9a to bring about silencing of the KCNQ1 locus [129–131]. It is a cis regulatory RNA, the aberration of which is associated with Beckwith-Wiedemann syndrome (a congenital overgrowth syndrome) [132, 133], colorectal cancer [129], hepatocellular carcinoma [134] and pediatric adrenocortical tumors [135].
ANRIL (Antisense Noncoding RNA at INK4 Locus), also known as p15AS, is an antisense transcript of CDKN2B gene at the 9p21.3 locus. It has several alternatively spliced isoforms including 3.9 kb and 34.8 kb transcripts [26, 136, 137]. Misexpression of ANRIL is associated with a variety of diseases including cancer [138–140]. ANRIL brings about changes in gene expression by epigenetic means as it binds to both PRC1 and PRC2 and mediates gene silencing at the INK4b-ARF-INK4a locus [26]. It specifically associates with SUZ12, (Suppressor of Zeste 12 homolog), a subunit of PRC2, and mediates the repression of p15, a tumor suppressor gene [26], and consequently inhibition of ANRIL induces p15, resulting in reduced cell proliferation.
XIST (X-inactive-specific transcript, ~17 kb), one the earliest lncRNAs to be discovered [27], is expressed mainly in female somatic cells. It is transcribed from the Xic (X inactivation Center) on the X chromosome and spreads along and coats the chromosome from which it is transcribed in order to epigenetically silence it in cis by recruiting PRC2 [72, 141–144], thus achieving dosage compensation in males. Deregulation of XIST leads to loss or gain of X chromosomes resulting in a variety of female, male and non sex specific cancers [145–147], demonstrating the participation of lncRNAs in maintaining genomic stability. In female cancers like breast, ovarian and cervical cancers, the inactive X chromosome (Xi or the Barr body) is conspicuously absent in the malignant cells, while its duplication was also observed in some cells [148], due to XIST deregulation. Further, a majority of female cancer cell lines exhibited multiple copies of the active X chromosome (Xa), which is acquired either due to duplication of Xa or due to reactivation of Xi [147]. In fact the aberrant expression of XIST results not only in aberrant ploidy of X chromosomes but also in the increased resistance of cancer cells to chemotherapy [145].
Interestingly, XIST is expressed in males also, specifically in the transcriptionally inactive XY body in spermatocytes [149], though it does not seem to be required for the inactivation of XY body since male mice lacking XIST undergo normal spermatogenesis and silencing of X linked genes [150]. Notably, Xist is known to be over-expressed in Testicular Germ Cell Tumors (TGCTs) and also in patients with Klinefelter’s syndrome (47XXY). In both these cases, super numerical X chromosomes were observed which is suggested to contribute to oncogenesis [151, 152]. Moreover, XIST RNA is detectable in the plasma of such patients and has emerged as a serum biomarker for both these disease conditions [153, 154]. X chromosomal duplications were also frequent in normal XY men with male breast cancer [155, 156].
Not only in male and female cancers, XIST is implicated in sex independent cancers as well, mainly in lymphomas and leukemias. Expression of XIST is lost is these cancers resulting in extra active X chromosomes in both male and female patients of non-Hodgkin lymphoma [157–159]. Thus, lncRNAs not only play an essential role in the regulation of individual genes but they also control the copy number of chromosomes as well.
HOTAIR (HOX Transcript Antisense Intergenic RNA) is a 2.2 kb lncRNA produced from the HOXC gene cluster on chromosome 12 (12q13.13) and is involved in the trans silencing of genes at HOXD locus on chromosome 2 [25, 77]. It provides a typical example of lncRNA regulation of gene expression through the chromatin remodelers. It serves as a scaffold to anchor multi-protein complexes and has a remarkable ability of binding to distinct chromatin repressors. Specifically, its 5’ end binds to PRC2 while its 3’ end binds to LSD1 (Lysine Specific Demethylase 1A), which in turn interacts with CoREST (Co-Repressor for Elements-1-Silencing Transcription factor) and REST (Repressor for Elements-1- Silencing Transcription factor), setting off long term epigenetic silencing of target chromatin region through H3K27Me3 mark [25, 77].
HOTAIR is known to repress several tumor and metastasis suppressor genes like HOXD10 (Homeobox D10), PGR (Progesterone Receptor), PCDH10 (Protocadherin10), PCDHB5 (Protocadherin Beta 5), JAM2 (Junctional Adhesion Molecule 2), etc. [160–162] and therefore its up-regulation leads to a variety of malignancies like primary/ metastatic breast cancers [161, 163–165], hepatocellular [166–168], colorectal [162], gastrointestinal [169, 170] and non-small cell lung carcinomas [171]. It is an oncogenic lncRNA associated with cell proliferation, invasiveness and reduced apoptosis and thus serves as a diagnostic and prognostic marker for multiple cancers.
While the above discussed lncRNAs are involved in gene regulation at epigenetic level, certain other lncRNAs are involved in transcriptional/ post transcriptional events, as exemplified by NEAT1 and MALAT1, the aberrant expression of which results in cancer.
NEAT1 (Nuclear Enriched Abundant Transcript 1) gene produces two transcripts, the 3.7 kb NEAT-1-1 short isoform and 23 kb NEAT-1-2 long isoform. NEAT1 is widely expressed across several tissues, though the expression of long isoform is much lower as compared to the short isoform. NEAT1 localizes to the paraspeckles in the nucleus [172, 173] and plays a crucial role in transcriptional and post-transcription regulation of gene expression and its knockdown leads to disintegration of paraspeckles [34]. In fact NEAT1 and NEAT2 (MALAT1) exhibit transcription dependent binding on human genome over hundreds of active genes. NEAT1 is induced strongly in breast cancer cells and is also involved in the transformation of myeloid cells into acute promyelocytic leukemia (APL) [174]. Further, it is highly upregulated in ATRA (All Trans Retinoic Acid) induced differentiation of NB4 (APL) cells which could be inhibited by specific siRNA for NEAT1 [174]. Silencing of NEAT1 in Burkitts lymphoma cells results in reduced viability, increased apoptosis and abnormal morphology suggesting its oncogenic nature [175].
MALAT1 (Metastasis Associated Lung Adenocarcinoma Transcript1) is another prominent lncRNA implicated in a variety of cancers. Also known as NEAT2 (Nuclear Enriched Abundant Transcript 2), it is a 7.5 kb RNA transcribed from the 11q13.1 locus, expressed broadly across various normal human tissues [67, 176]. MALAT1 undergoes post-transcriptional processing to yield a short t-RNA like cytoplasmic mascRNA (malat1 associated small cytoplasmic RNA) and a long MALAT1 transcript that localizes to nuclear speckles and is involved in splicing events. It specifically localizes to the nuclear speckles of SR (Serine Arginine) proteins, which are required for both constitutive and alternative splicing and the levels of MALAT1 directly influence the level of phosphorylated SR proteins [29, 67]. It is over-expressed in a variety of cancers like lung [176, 177], liver [178, 179], bladder [180, 181], pancreatic [182], cervical [183], breast [184], prostate [185], colorectal [186] and uterus [187]. It is specifically linked to high metastasis rate and poor prognosis in non-small cell lung cancer patients [188]. Further, its overexpression is shown to bring about cell survival, proliferation, migration and promotion of epithelial-mesenchymal transition by activating Wnt signaling in vitro in urothelial carcinoma [180, 181] and hence it is suggested to be involved in cell motility. Notably, while the over expression of MALAT1 is associated with severe consequences, its knockdown in mice is neither lethal nor shown to cause any defects in vivo [189].
Certain lncRNAs like SRA and GAS5 mediate gene regulation through interaction with hormone receptors and lead to cancer when deregulated, as discussed below.
SRA (Steroid receptor RNA Activator), an lnc RNA transcribed from the 5q31.3 locus, 2 kb in size, is a coactivator of various steroid hormone receptors as discussed earlier. It has been reported that the SRA1 gene plays a dual role and codes both for a protein (SRAP) and an lncRNA (SRA), by alternative splicing [190–192]. The levels of this protein and the lncRNA are suggested to impact tumorigenesis and tumor progression by varying the expression of target genes [98]. SRA is a part of RNA-protein (RNP) complex and brings about the trans activation of genes through its interaction with the AF1 domain of nuclear receptors [69]. Its over-expression and consequent deregulated hormone signaling is associated with breast [193, 194], uterine, ovarian [195] and prostate [190] cancers.
GAS5 (Growth Arrest Specific 5) gene at 1q25.1 locus produces two splice variant lncRNAs and its introns also give rise to several snoRNAs [196]. GAS5 functions as a tumor suppressor and facilitates normal growth arrest and apoptosis through repression of GR mediated transcription [22, 197]. It specifically interacts with DNA binding domain of GR and inhibits the binding of GR to its target genes including cIAP2 (cellular Inhhibitor of Apoptosis 2), bringing about apoptosis, independent of other stimuli in cancer cells. Moreover, GAS5 has also been suggested to repress progesterone receptor and androgen receptor in a ligand dependent fashion [22, 196]. It also mediates the inhibition of mTOR (mammalian Target of Rapamycin), which regulates protein synthesis, cell growth and proliferation. This fact is corroborated by the observation that anti proliferative effect induced by Rapamycin could be repressed by silencing of GAS5 in primary T cells as well as in leukemic cells [198]. In turn, GAS5 is regulated by a negative feedback loop with miR-21 [199]. Down-regulation of GAS5 and/or its snoRNAs along with genetic aberrations at its locus were found to be associated with poor prognosis in several cancers like breast cancer [197, 200], prostate cancer [201], leukemia [198], gastric cancer [202], cervical cancer [203], renal cell and Bladder cancer [204, 205].
Telomere associated lncRNA, TERRA in human cancers
Telomeres, the ends of chromosomes, are composed of a hexanucleotide repeat, TTAGGG in vertebrates which protects and prevents end to end fusion in chromosomes [206]. The telomere repeats shorten after each cell cycle in normal cells which can lead to chromosome instability and cell death [207]. Most of the cancer cells overcome this adversity by Telomerase activity which requires ncRNAs. The telomerase enzyme has a protein component called TERT (Telomerase Reverse Transcriptase) and an RNA component called TERC (Telomerase RNA Component) [208]. Apart from TERC, a group of lncRNA transcripts named TERRA (Telomeric Repeat containing RNA) derived from the subtelomeric loci has recently been identified. TERRA localizes to and brings about the hetrochromatin formation in telomeres, a conserved phenomenon in eukaryotic cells [209, 210]. TERRA is suggested to be a negative regulator of telomerase and leads to cancer when down-regulated [211, 212].
T-UCRs in human cancers
Transcribed Ultraconserved Regions (T-UCRs) are evolutionary highly conserved sequences between orthologous regions of the human, rat and mouse genomes [213, 214]. They give rise to transcripts of 200–779 nt in length that show tissue specific expression. Many of the T-UCRs show altered expression in cancers like chronic lymphocytic leukemia [214], colorectal carcinoma [214], neuroblastomas [215, 216], hepatocellular carcinoma [217] and prostate cancer [218]. The T-UCRs can be targeted by miRNAs and offer opportunities for novel therapeutic interventions [214, 219].
While the above mentioned lncRNAs are implicated in multiple cancers, certain other lncRNAs have so far been linked only to specific cancer types so far as discussed below.
HULC (Highly Up-regulated in Liver Cancer), 1.6 kb in size, is transcribed from the 6p23.3 locus. It was discovered by Panzitt et al. [220] with the help of Hepato Cellular Carcinoma (HCC) specific microarray as the most highly up-regulated lncRNA in this cancer. Like a typical mRNA, it has two exons and a poly A tail and strongly localizes to the cytoplasm and co-purifies with ribosomes but does not code for any protein. It sequesters miRNAs and is involved in the inhibition of miRNA mediated repression. Liu et al. [221] reported that the SNP, rs7763881 in HULC locus was significantly associated with HCC susceptibility in HBV (Hepatitis B Virus) carriers. Further, knockdown of CREB (cAMP response element-binding protein) expression as well as use of a PKA (Protein kinase A) inhibitor resulted in down regulation of HULC, revealing that phospho CREB is required for activation of HULC [222]. HULC is oncogenic in nature and highly up-regulated in both tumors and plasma of HCC patients but it is not detected in any other tissues or their cancers [220]. Thus it serves as a specific non-invasive biomarker for HCC [223]. Moreover, it is not expressed in primary colorectal cancers but is detected in colorectal cancers that metastasize to liver showing its specificity for the hepatic tissue [224].
HEIH (High Expression In HCC) is a 1.6 kb oncogenic polyadenylated transcript generated from the 5q34.3 locus. Yang et al. [225] examined the differentially expressed lncRNAs between HBV related HCC and normal tissues and one of the RNAs, HEIH, was studied in detail. HEIH was shown to play a critical role during cell cycle and associated with EZH2 (Enhancer of Zeste Homolog 2), a critical component of PRC2 and represses the EZH2 target genes [225]. The levels of HEIH were found to be significantly associated with HCC recurrence and post-operative survival of patients and thus it serves as an independent prognostic factor [225].
PCA3 (Prostate Cancer Antigen 3; also known as DD3, Differential Display Code3) is derived from the 9q21.22 locus, and transcribed as three alternately spliced isoforms of 0.6 kb, 2 kb and 4 kb [226]. It is expressed at low levels in normal prostate and highly up-regulated in >95 % of prostate tumors, but not in any other normal or cancer tissue. It is a potent biomarker detectable in urine of prostate cancer patients with higher specificity and sensitivity as compared to PSA (Prostate Specific Antigen) [226, 227].
PCGEM1 (Prostate Cancer Gene Expression Marker), 1.6 kb in size, derived from the 2q32 locus, it is one of the earliest oncogenic lncRNAs discovered. It is regulated by Androgen Receptor (AR), a transcription factor which has a critical role in the prostate gland development [228]. PCGEM1 is highly elevated in prostate cancer, especially in patients with a family history of prostate cancer. It promotes cell growth, proliferation and inhibits doxorubicin induced apoptosis. Overexpression of PCGEM inhibits PARP cleavage and delays the induction of p53 and p21 resulting in increased chemo-resistance. It plays an important role during carcinogenesis and serves as a specific biomarker for prostate cancer [229].
PCAT1 (Prostate Cancer Associated ncRNA Transcript 1) is a 7.8 kb lncRNA transcribed from the 8q24.13 locus, up-regulated in both metastatic and high grade localized prostate tumors. Prensner et al. [230] identified 121 prostate cancer associated transcripts (PCATs) by RNA sequencing analysis of prostate cancer tissues of which PCAT1 is the most highly up-regulated. Knock down of PCAT1 in androgen dependent prostate cancer cell line resulted in alteration of hundreds of genes [230]. It has also been reported that PCAT1 has an important role to play in double strand break repair and inhibits homologous recombination [231]. It is a transcriptional repressor of DNA repair genes like BRCA2 tumor suppressor and in turn is regulated by PRC2. Overexpression of PCAT1 is linked to increased sensitivity to PARP inhibitors due to decrease in RAD51 foci formation [231]. PCAT1 is a negative prognostic marker for prostate cancer [230].
These prostate specific lncRNAs are proving to be very useful in the clinic as diagnostic and prognostic markers in Prostate cancer since the traditional markers like PSA have only limited prognostic value [232].
Anti-NOS2A (Anti Nitric Oxide Synthase 2A) is a 1.9 kb intronless polyadenylated lncRNA expressed in meningomas and glioblastomas, transcribed from the NOS2A (17q23.2) locus. It is involved in the negative regulation of NOS2A, which plays an important role in the neuronal differentiation [233].
HOTAIRM1 is a 483 bp transcript generated from the HOXA cluster. It is a regulator of hematopoiesis and its down-regulation results in the inhibition of several HOXA genes required for hematopoiesis. In APL (Acute Promyelocytic Leukemia), differentiation of hematopoietic precursors gets blocked at promyelocytic stage due to chromosomal translocations involving Retinoic Acid Receptor alpha (RAR α) gene. ATRA (All Trans Retinoic Acid) is used to treat this condition and HOTAIRM1 was found to be induced in ATRA mediated differentiation of APL cells [234], showing that its down regulation is linked to the disease phenotype.
DLEU1 and DLEU2 (Deleted in lymphocytic Leukemia 1 and 2) are two lncRNAs produced from the 13q14.3 tumor suppressor locus [235] which is deleted in lymphomas and hematopoitic cancers like Chronic Lymphocytic Leukemia (CLL) [236, 237]. DLEU1 and DLEU2 are regulated epigenetically and in turn regulate a cluster of genes that influence NF-kB expression. Interestingly, expression of the protein coding genes at the 13q14.3 locus is altered but they are not associated with any SNPs, whereas the promoter regions of the two lncRNAs exhibit demethylation/activation marks in CLL suggesting that the lncRNAs regulate the protein coding genes in cis. Further, DLEU2 splice variants are the precursors of cell cycle inhibitory miRNAs, miR-15a and miR-16-1, which are suggested to be involved in CLL [238, 239].
Apart from the individual lncRNAs associated with cancer, several genome wide microarray analyses in recent years have shed light upon hundreds or thousands of lncRNAs that are deregulated in various cancers [240–244], further corroborating the fact that lncRNAs are important players involved in the development and progression of cancers.
LncRNAs as potential biomarkers and therapeutic targets
LncRNAs are not only providing us with a new perspective to our understanding of disease mechanisms but also furnishing fresh therapeutic opportunities [245–247]. In fact lncRNAs have an advantage over protein coding genes in that their expression is more tissue specific, thus making them attractive as biomarkers and therapeutic targets. LncRNAs are remarkably stable in body fluids and tissues, proving to be valuable biomarkers in liquid biopsies, facilitating the avoidance of invasive procedures [105, 248, 249]. Their distribution and levels can be evaluated with the help of various techniques like in situ hybridization, qPCR, transcriptome profiling etc. [248], which can be used to assess the disease progression and/or recovery with a particular treatment regimen.
LncRNAs can be targeted therapeutically by a variety of approaches including RNAi mediated gene silencing, antisense oligonucleotides, plasmid based targeting, through small molecule inhibitors and by gene therapy as discussed below (Reviewed in 105, [250–252].
RNAi mediated down regulation of specific lncRNAs for therapy
RNA interference mediated silencing of genes involved in various diseases provides a direct approach to selectively inhibit target molecules. This can be achieved through different agents like siRNA (small interfering RNAs), shRNAs (short hairpin RNAs), and miRNAs. Even though most of the lncRNAs are known to show nuclear localization, various studies have revealed that they can still be targeted by RNAi mediated intervention [39].
The lncRNA HOTAIR is upregulated and serves as a diagnostic and prognostic biomarker for breast, liver, gastro-intestinal, lung and colorectal carcinomas [161–171, 253]. Down regulation of HOTAIR expression by siRNA is associated with reduced viability and invasiveness and induction of apoptosis in breast, hepatocellular and pancreatic cancers [167, 254, 255]. Furthermore, knockdown of HOTAIR also enhanced the sensitivity of cancer cells to tumor necrosis factor alpha based immune response and also to chemotherapeutic agents like cisplatin and doxorubicin [167]. The lncRNA PCA3 is highly up-regulated in prostate cancer and is a potent biomarker detectable in urine [226]. siRNA mediated down-regulation of PCA3 significantly inhibited growth and viability of prostate cancer cells and also reduced the expression of AR target genes [256], suggesting it can be a potential therapeutic target. LncRNAs PCAT1, PRNCR1, PCGEM, PlncRNA1 and PCAT18 are also highly up-regulated in aggressive prostate tumors and have been suggested as biomarkers and therapeutic targets for the same [257, 258]. siRNA/shRNA based silencing of these lncRNAs in prostate cancer cell lines inhibited cell proliferation and induced apoptosis by decrease in AR expression [258, 259]. The lncRNAs H19, HULC, HEIH, MVIH are highly upregulated in hepatocellular cancer and are valuable biomarkers for the same [116, 220, 225, 260]. siRNA/shRNA mediated silencing of these transcripts resulted in altered expression of several genes and reduced growth of tumors in xenografts indicating they are potential therapeutic targets [220, 225]. The lncRNAs H19, UCA1, CUDR, HIF1A-AS are reliable biomarkers and potential therapeutic targets for bladder cancer [123, 261–265]. MALAT1 is a prognostic marker for lung, gastrointestinal and several other cancers [176–188]. shRNA mediated silencing of MALAT1 inhibited the migration and invasive potential of adenocarcinoma cells and cervical cancer cells respectively [177, 178]. Down-regulation of MALAT1 by siRNA in HEPG2 cell line results in reduction in tumor progression, cell motility and viability along with induction of apoptosis [179]. The lncRNA CCAT2 is up-regulated in colorectal cancer and can be targeted by specific miRNAs [Table 1].
Although RNAi based therapeutic agents are used to target lncRNAs in cell lines quite effortlessly, in vivo, they would require suitable delivery vehicles like liposomes, nanoparticles or viruses for proper cellular uptake, prevention of their degradation or accumulation in liver. Nevertheless, several RNAi based therapies are in clinical trials [266, 267], though there is still a need for further improvements for safe and effective remedies.
Antisense Oligonucleotides (ASO) mediated therapy
Antisense oligonucleotides are short (13–25) single stranded DNA oligonucleotides complementary to RNA of interest. ASOs are modified to avoid degradation by nucleases and in turn they induce RNase H mediated cleavage of their target transcripts. Several ASOs, mainly those targeting mRNAs are already in advanced clinical trials [268, 269], while two ASO based drugs to treat Cytomegalovirus retinitis and high blood cholesterol have already been approved by FDA [270, 271]. Similar approaches are being developed to target cancer related lncRNAS. Accordingly, AntagoNATs, ASOs that target antisense lncRNA, are being employed to up-regulate specific mRNAs/ proteins by silencing the corresponding antisense lncRNA [272]. AntagoNATs are modified not only in their 5’ and 3’ termini but also in their backbone in order to make them more stable and to enhance their cellular uptake. Thus ASOs have an advantage over siRNAs which are usually unstable and hard to be targeted into tumor cells in vivo [273]. Notably, ASO (Antisense Oligonucleotide) mediated knockdown of MALAT1 inhibited the metastasis in human lung cancer cells in a mouse xenograft model [188]. Despite the promise, poor cellular uptake and cytotoxicity remain as matters of concern for ASOs.
Small molecule inhibitor mediated modulation of lncRNAs
The molecular interactions of lncRNAs with their interacting protein partners can be blocked by small molecule inhibitors that mask the binding sites on their interactors [251]. Accordingly, the interaction of HOTAIR with PRC2 or LSD1 can be inhibited with the help of small molecular inhibitors to reduce the metastasis in breast cancer [274]. Alternately, in another approach, small molecule inhibitors or specific oligonucleotides can be designed to bind and change the secondary structure of lncRNAs and thus inhibit their interaction with binding partners [251, 275]. Targeting the lncRNA-protein interactions would not only lend tissue and developmental specificity but also has an advantage over targeting only RNAs or proteins since lncRNAS mediate regulation of gene expression essentially through their protein partners. Furthermore, this method is also superior to RNAi based methods which may suffer from off target effects. Moreover small molecules are easier to be administered and exhibit a better cellular uptake than ASOs, siRNAs or viral vectors. However, this approach needs a better understanding of RNA-protein interactions and identification of additional molecules that target RNA.
Plasmid based therapy
In a novel therapeutic approach, a plasmid, BC-819/ DTAH19, has been developed which carries a diphtheria toxin subunit under the regulation of H19 promoter. When this plasmid is injected into the tumor, it brings about the reduction in tumor size due to the production of high level of diphtheria toxin in human trials of bladder cancer [276]. This method attempts to reduce the tumor size in general rather than targeting any specific lncRNA and has shown encouraging results in recent times in other cancers like lung, colon, pancreatic and ovarian cancer as well [101].
Gene therapy
Some lncRNAs are down-regulated in tumor samples as compared to normal tissues. The lncRNA PTCSC3 (Papillary Thyroid Carcinoma Susceptibility Candidate 3) is down-regulated in thyroid tumors [277]. PTENP1, a pseudogene of PTEN, is down-regulated in colon carcinoma [278]. MEG3 is downregulated in meningioma and glioma [279]. LincRNA-p21 is down-regulated in lymphoma, lung carcinoma [56] and colorectal cancer [280]. Delivery of beneficial tumor suppressor RNAs can be attempted with the help of gene therapy in such cases [247, 251].
In summary, though the above discussed means of targeting long noncoding RNAs for cancer therapy looks very promising in cell lines, the delivery of therapeutic agents to their specific targets in actual patients in vivo would be quite challenging and effective strategies need to be developed for the same [281]. Although, trials on mouse models have shown some hope, but many of the lncRNAs are primate/ human specific and cannot be investigated in vivo in knockdown/ knockout models in mice. Another point of concern is the fact that even though it has been well established that altered expression of lncRNAs is associated with various cancers, it has not yet been clearly recognized whether the alteration is a cause or consequence of the disease. This calls for a thorough understanding of structure and mechanism of lncRNAs, their molecular interactions and development of novel quantitative assays to screen for drugs. Nonetheless, lncRNAs offer new hope for novel treatment options and in the near future it is expected that many of the lncRNAs may end up as strong diagnostic tools for cancer detection and patient management in the clinic. Because of the increasing number of cancer cases and its incurable nature, there is always a need for novel biomarkers for diagnosis, prognosis and therapy.
Key oncogenic and tumor suppressor lncRNAs suggested as potential biomarkers/therapeutic targets are summarized in Table 1.
Conclusions and future perspectives
Cancer, being an incurable disease so far, needs novel and effective biomarkers and therapeutic strategies. It is becoming increasingly apparent that deregulated lncRNAs form a new stratum of intricacy in the molecular makeup of human diseases. Their role and mode of action in various signaling pathways during normal and disease conditions is being dissected meticulously and their significance is being acknowledged widely. LncRNAs are strongly associated with clinico-pathological outcome and prognosis of various diseases, more particularly in cancers and furnishing fresh therapeutic possibilities. They are generally expressed in tissue specific manner and exhibit aberrant expression in cancers. Therefore, targeting and either down-regulating or up-regulating specific lncRNAs in malignancies may not have deleterious side effects on normal cells. Thus, of late, both academia and biotech companies are turning their attention towards these novel and possibly personalized treatment options and trying to develop biological/nucleic acid drugs [282].
Various companies/organizations like RaNa Therapeutics, CuRNA, Sarepta, Smart Therapeutics, Allen Institution for Brain Science, Regulus, Miragen Therapeutics, Santaris Pharma etc. are pioneering the ncRNA based medicines. Soon we may see the time when lncRNA signature becomes a routine diagnostic test for diverse diseases, followed up by RNA based therapy curing the hitherto incurable diseases like cancer.
References
Gilbert W. Origin of life—The RNA world. Nature. 1986;319:618–8.
Lander ES, Linton LM, Birren B, Nusbaum C, Zody MC, Baldwin J, et al. Initial sequencing and analysis of the human genome. Nature. 2001;409:860–921.
Human Genome Sequencing ConsortiumInternational. Finishing the euchromatic sequence of the human genome. Nature. 2004;431:931–45.
The ENCODE Project Consortium. An integrated encyclopedia of DNA elements in the human genome. Nature. 2012;489:57–74.
Okazaki Y, Furuno M, Kasukawa T, Adachi J, Bono H, Kondo S, et al. Analysis of the mouse transcriptome based on functional annotation of 60,770 full-length cDNAs. Nature. 2002;420:563–73.
Lodish H, Berk A, Zipursky SL, Matsudaira P, Baltimore D, Darnell J. The Three Roles of RNA in Protein Synthesis. 2000.
Morris KV, Mattick JS. The rise of regulatory RNA. Nat Rev Genet. 2014;15:423–37.
Farazi TA, Juranek SA, Tuschl T. The growing catalog of smallRNAs and their association with distinct Argonaute/Piwi family members. Development. 2008;135:1201–14.
Wang X, Song X, Glass CK, Rosenfeld MG. The long arm of longnoncoding RNAs: roles as sensors regulating gene transcriptional programs. Cold Spring Harb Perspect Biol. 2011;3:a003756. doi:10.1101/cshperspect.a003756.
Frith MC, Forrest AR, Nourbakhsh E, Pang KC, Kai C, Kawai J, et al. The abundance of short proteins in the mammalian proteome. PLoS Genet. 2006;2, e52.
Guttman M, Amit I, Garber M, French C, Lin MF, Feldser D, et al. Chromatin signature reveals over a thousand highly conserved large non-coding RNAs in mammals. Nature. 2009;458:223–7.
Derrien T, Johnson R, Bussotti G, Tanzer A, Djebali S, Tilgner H, et al. The GENCODE v7 catalog of human long noncoding RNAs: Analysis of their gene structure, evolution, and expression. Genome Res. 2012;22:1775–89.
Kapranov P, Cheng J, Dike S, Nix DA, Duttagupta R, Willingham AT, et al. RNA maps reveal new RNA classes and a possible function for pervasive transcription. Science. 2007;316:1484–8.
Niazi F, Valadkhan S. Computational analysis of functional long noncoding rnas reveals lack of peptide-coding capacity and parallels with 3' UTRs. RNA. 2012;18:825–43.
Wan Y, Qu K, Ouyang Z, Kertesz M, Li J, Tibshirani R, et al. Genome-wide measurement of RNA folding energies. Mol Cell. 2012;48:169–81.
Louro R, Smirnova AS, Verjovski-Almeida S. Long intronic non coding RNA transcription: expression noise or expression choice? Genomics. 2009;93:291–8.
Mercer TR, Dinger ME, Mattick JS. Long non-coding RNAs: insights into functions. Nat Rev Genet. 2009;10:155–9.
Pang KC, Frith MC, Mattick JS. Rapid evolution of non-coding RNAs: lack of conservation does not mean lack of function. Trends Genet. 2006;22:1–5.
Nagano T, Fraser P. No-nonsense functions for long noncoding RNAs. Cell. 2011;145:178–81.
Geisler S, Coller J. RNA in unexpected places: long non-coding RNA functions in diverse cellular contexts. Nat Rev Mol Cell Biol. 2013;14:699–712.
Wang KC, Chang HY. Molecular mechanisms of long noncoding RNAs. Mol Cell. 2011;43:904–14.
Kino T, Hurt DE, Ichijo T, Nader N, Chrousos GP. Noncoding RNA gas5 is a growth arrest- and starvation-associated repressor of the glucocorticoid receptor. Sci Signal. 2010;3:ra8. doi:10.1126/scisignal.2000568.
Brown JA, Valenstein ML, Yario TA, Tycowski KT, Steitz JA. Formation of triple-helical structures by the 3’-end sequences of MALAT1 and MENβ noncoding RNAs. Proc Natl Acad Sci U S A. 2012;109:19202–7.
Bak RO, Mikkelsen JG. miRNA sponges: soaking up miRNAs for regulation of gene expression. Wiley Interdiscip Rev RNA. 2014;5:317–33.
Tsai MC, Manor O, Wan Y, Mosammaparast N, Wang JK, Lan F, et al. Long noncoding RNA as modular scaffold of histone modification complexes. Science. 2010;329:689–93.
Kotake Y, Nakagawa T, Kitagawa K, Suzuki S, Liu N, Kitagawa M, et al. Long non-coding RNA anril is required for the PRC2 recruitment to and silencing of p15(INK4B) tumor suppressor gene. Oncogene. 2011;30:1956–62.
Lee JT. Lessons from X-chromosome inactivation: long ncRNA as guides and tethers to the epigenome. Genes Dev. 2009;23:1831–42.
Yoon JH, Abdelmohsen K, Srikantan S, Yang X, Martindale JL, De S, et al. LincRNAp21 suppresses target mRNA translation. Mol Cell. 2012;47:648–55.
Tripathi V, Ellis JD, Shen Z, Song DY, Pan Q, Watt AT, et al. The nuclear-retained noncoding RNA MALAT1 regulates alternative splicing by modulating SR splicing factor phosphorylation. Mol Cell. 2010;39:925–38.
Gong C, Maquat LE. lncRNAs transactivate STAU1-mediated mRNA decay by duplexing with 3’ UTRs via Alu elements. Nature. 2011;470:284–8.
Affymetrix ENCODE Transcriptome Project and Cold Spring Harbor Laboratory ENCODE Transcriptome Project. Post-transcriptional processing generates a diversity of 50-modified long and short RNAs. Nature. 2009;457:1028–32.
Mattick JS, Makunin IV. Small regulatory RNAs in mammals. Hum Mol Genet. 2005;14:R121–32.
Ha H, Song J, Wang S, Kapusta A, Feschotte C, Chen KC, et al. A comprehensive analysis of piRNAs from adult human testis and their relationship with genes and mobile elements. BMC Genomics. 2014;15:545.
Clemson CM, Hutchinson JN, Sara SA, Ensminger AW, Fox AH, Chess A, et al. An architectural role for a nuclear noncoding RNA: NEAT1 RNA is essential for the structure of paraspeckles. Mol Cell. 2009;33:717–26.
Hacisuleyman E, Goff LA, Trapnell C, Williams A, Henao-Mejia J, Sun L, et al. Topological organization of multichromosomal regions by the long intergenic noncoding RNA Firre. Nat Struct Mol Biol. 2014;21:198–206.
Wood AJ, Oakey RJ. Genomic imprinting in mammals: emerging themes and established theories. PLoS Genet. 2006;2, e147.
Bartolomei MS. Genomic imprinting: employing and avoiding epigenetic processes. Genes Dev. 2009;23:2124–33.
Wan L-B, Bartolomei MS. Regulation of imprinting in clusters: noncoding RNAs versus insulators. Adv Genet. 2008;61:207–23.
Khalil AM, Guttman M, Huarte M, Garber M, Raj A, Rivea Morales D, et al. Many human large intergenic noncoding RNAs associate with chromatin-modifying complexes and affect gene expression. Proc Natl Acad Sci U S A. 2009;106:11667–72.
Cao R, Wang L, Wang H, Xia L, Erdjument-Bromage H, Tempst P, et al. Role of histone H3 lysine 27 methylation in Polycomb-group silencing. Science. 2002;298:1039–43.
Mattick JS, Gagen MJ. Review Article The Evolution of Controlled Multitasked Gene Networks : The Role of Introns and Other Noncoding RNAs in the Development of Complex Organisms. Mol Biol Evol. 2001;18:1611–30.
Mattick JS. The genetic signatures of noncoding RNAs. PLoS Genet. 2009;5, e1000459.
Margueron R, Reinberg D. The Polycomb complex PRC2 and its mark in life. Nature. 2011;469:343–9.
Lakowski B, Roelens I, Jacob S. CoREST-like complexes regulate chromatin modification and neuronal gene expression. J Mol Neurosci. 2006;29:227–39.
Tahiliani M, Mei P, Fang R, Leonor T, Rutenberg M, Shimizu F, et al. The histone H3K4 demethylase SMCX links REST target genes to X-linked mental retardation. Nature. 2007;447:601–5.
Tachibana M, Matsumura Y, Fukuda M, Kimura H, Shinkai Y. G9a/GLP complexes independently mediate H3K9 and DNA methylation to silence transcription. EMBO J. 2008;27:2681–90.
Shi Y-J, Matson C, Lan F, Iwase S, Baba T, Shi Y. Regulation of LSD1 Histone Demethylase Activity by Its Associated Factors. Mol Cell. 2005;19:857–64.
Schuettengruber B, Chourrout D, Vervoort M, Leblanc B, Cavalli G. Genome regulation by polycomb and trithorax proteins. Cell. 2007;128:735–45.
Seila AC, Calabrese JM, Levine SS, Yeo GW, Rahl PB, Flynn RA, et al. Divergent transcription from active promoters. Science. 2008;322:1849–51.
Core LJ, Waterfall JJ, Lis JT. Nascent RNA sequencing reveals widespread pausing and divergent initiation at human promoters. Science. 2008;322:1845–8.
Taft RJ, Glazov EA, Cloonan N, Simons C, Stephen S, Faulkner GJ, et al. Tiny RNAs associated with transcription start sites in animals. Nat Genet. 2009;41:572–8.
Wang X, Arai S, Song X, Reichart D, Du K, Pascual G, et al. Induced ncRNAs allosterically modify RNA-binding proteins in cis to inhibit transcription. Nature. 2008;454:126–30.
Hung T, Wang Y, Lin MF, Koegel AK, Kotake Y, Grant GD, et al. Extensive and coordinated transcription of noncoding RNAs within cell-cycle promoters. Nat Genet. 2011;43:621–9.
Negishi M, Wongpalee SP, Sarkar S, Park J, Lee KY, Shibata Y, et al. A new lncRNA, APTR, associates with and represses the CDKN1A/p21 promoter by recruiting polycomb proteins. PLoS One. 2014;9, e95216.
Dimitrova N, Zamudio JR, Jong RM, Soukup D, Resnick R, Sarma K, et al. LincRNA-p21 activates p21 in cis to promote Polycomb target gene expression and to enforce the G1/S checkpoint. Mol Cell. 2014;54:777–90.
Huarte M, Guttman M, Feldser D, Garber M, Koziol MJ, Kenzelmann-Broz D, et al. A large intergenic noncoding RNA induced by p53 mediates global gene repression in the p53 response. Cell. 2010;142:409–19.
Bulger M, Groudine M. Functional and mechanistic diversity of distal transcription enhancers. Cell. 2011;144:327–39.
Shen Y, Yue F, McCleary DF, Ye Z, Edsall L, Kuan S, et al. A map of the cis-regulatory sequences in the mouse genome. Nature. 2012;488:116–20.
Ørom UA, Derrien T, Beringer M, Gumireddy K, Gardini A, Bussotti G, et al. Long noncoding RNAs with enhancer-like function in human cells. Cell. 2010;143:46–58.
Kim T-K, Hemberg M, Gray JM, Costa AM, Bear DM, Wu J, et al. Widespread transcription at neuronal activity-regulated enhancers. Nature. 2010;465:182–7.
Lai F, Orom UA, Cesaroni M, Beringer M, Taatjes DJ, Blobel GA, et al. Activating RNAs associate with Mediator to enhance chromatin architecture and transcription. Nature. 2013;494:497–501.
Mousavi K, Zare H, Dell’orso S, Grontved L, Gutierrez-Cruz G, Derfoul A, et al. eRNAs promote transcription by establishing chromatin accessibility at defined genomic loci. Mol Cell. 2013;51:606–17.
Shibayama Y, Fanucchi S, Magagula L, Mhlanga MM. lncRNA and gene looping. Transcription. 2014;5, e28658.
Lovén J, Hoke HA, Lin CY, Lau A, Orlando DA, Vakoc CR, et al. Selective inhibition of tumor oncogenes by disruption of super-enhancers. Cell. 2013;153:320–34.
Whyte WA, Orlando DA, Hnisz D, Abraham BJ, Lin CY, Kagey MH, et al. Master transcription factors and mediator establish super-enhancers at key cell identity genes. Cell. 2013;153:307–19.
Xiang J-F, Yin Q-F, Chen T, Zhang Y, Zhang X-O, Wu Z, et al. Human colorectal cancer-specific CCAT1-L lncRNA regulates long-range chromatin interactions at the MYC locus. Cell Res. 2014;24:513–31.
Hutchinson JN, Ensminger AW, Clemson CM, Lynch CR, Lawrence JB, Chess A. A screen for nuclear transcripts identifies two linked noncoding RNAs associated with SC35 splicing domains. BMC Genomics. 2007;8:39.
Yoon J-H, Abdelmohsen K, Gorospe M. Posttranscriptional gene regulation by long noncoding RNA. J Mol Biol. 2013;425:3723–30.
Lanz RB, McKenna NJ, Onate SA, Albrecht U, Wong J, Tsai SY, et al. A steroid receptor coactivator, SRA, functions as an RNA and is present in an SRC-1 complex. Cell. 1999;97:17–27.
Kitagawa M, Kotake Y, Ohhata T. Long non-coding RNAs involved in cancer development and cell fate determination. Curr Drug Targets. 2012;13:1616–21.
Di Gesualdo F, Capaccioli S, Lulli M. A pathophysiological view of the long non-coding RNA world. Oncotarget. 2014;5:10976–96.
Brown CJ, Hendrich BD, Rupert JL, Lafreniere RG, Xing Y, Lawrence J, et al. The Human XIST Gene: Analysis of a 17 kb inactive X-specific RNA that contains conserved repeats and is highly localized within the nucleus. Cell. 1992;71:527–42.
Zhao J, Sun BK, Erwin JA, Song JJ, Lee JT. Polycomb proteins targeted by a short repeat RNA to the mouse X chromosome. Science. 2008;332:750–6.
Lee JT, Lu N. Targeted mutagenesis of Tsix leads to nonrandom X inactivation. Cell. 1999;99:47–57.
Tian D, Sun S, Lee JT. The long noncoding RNA, Jpx, is a molecular switch for X-chromosome inactivation. Cell. 2010;143:390–403.
Marahrens Y, Panning B, Dausman J, Strauss W, Jaenisch R. Xist-deficient mice are defective in dosage compensation but not spermatogenesis. Genes Dev. 1997;11:156–66.
Rinn JL, Kertesz M, Wang JK, Squazzo SL, Xu X, Brugmann SA, et al. Functional demarcation of active and silent chromatin domains in human HOX loci by non-coding RNAs. Cell. 2007;129:1311–23.
Wang KC, Yang YW, Liu B, Sanyal A, Corces-Zimmerman R, Chen Y, et al. A long noncoding RNA maintains active chromoatin to coordinate homeotic gene expression. Nature. 2011;472:120–4.
Li L, Liu B, Wapinski OL, Tsai MC, Qu K, Zhang J, et al. Targeted disruption of Hotair leads to homeotic transformation and gene derepression. Cell Reports. 2013;5:3–12.
Sleutels F, Zwart R, Barlow DP. The non-coding Air RNA is required for silencing autosomal imprinted genes. Nature. 2002;415:810–3.
Nagano T, Mitchell JA, Sanz LA, Pauler FM, Ferguson-Smith AC, Feil R, et al. The Air noncoding RNA epigenetically silences transcription by targeting G9a to chromatin. Science Reports. 2008;322:1717–20.
Mohammad F, Mondal T, Kanduri C. Epigenetics of imprinted long noncoding RNAs. Epigenetics. 2009;4:277–86.
Kanduri C. Kcnq1ot1: A chromatin regulatory RNA. Sem Cell Dev Biol. 2011;22:343–50.
Bartolomei MS, Zemel S, Tilghman SM. Parental imprinting of the mouse H19 gene. Nature. 1991;351:153–5.
Ripoche MA, Kress C, Poirier F, Dandolo L. Deletion of the H19 transcription unit reveals the existence of a putative imprinting control element. Genes Dev. 1997;11:1596–604.
Monnier P, Martinet C, Pontis J, Stancheva I, Ait-Si-Ali S, Dandolo L. H19 lncRNA controls gene expression of the Imprinted Gene Network by recruiting MBD1. PNAS. 2013;110:20693–8.
Loewer S, Cabili MN, Guttman M, Loh YH, Thomas K, Park IH, et al. Large intergenic non-coding RNA-RoR modulates reprogramming of human induced pluripotent stem cells. Nat Genet. 2010;12:1113–7.
Kretz M, Webster DE, Flockhart RJ, Lee CS, Zehnder A, Lopez-Pajares V, et al. Suppression of progenitor differentiation requires the long noncoding RNA ANCR. Genes Dev. 2012;26:338–43.
Cesana M, Cacchiarelli D, Legnini I, Santini T, Sthandier O, Chinappi M, et al. A long noncoding RNA controls muscle differentiation by functioning as a competing endogenous RNA. Cell. 2011;147:358–69.
Klattenhoff CA, Scheuermann JC, Surface LE, Bradley RK, Fields PA, Steinhauser ML, et al. Braveheart, a long noncoding RNA required for cardiovascular lineage commitment. Cell. 2013;152:570–83.
Berghoff EG, Clark MF, Chen S, Cajigas I, Leib DE, Kohtz JD. Evf2 (Dlx6as) lncRNA regulates ultraconserved enhancer methylation and the differential transcriptional control of adjacent genes. Development. 2013;140:4407–16.
Ng SY, Johnson R, Stanton LW. Human long non-coding RNAs promote pluripotency and neuronal differentiation by association with chromatin modifiers and transcription factors. EMBO J. 2012;31:522–33.
Shore AN, Kabotyanski EB, Roarty K, Smith MA, Zhang Y, Creighton CJ, et al. Pregnancy-Induced Noncoding RNA (PINC) Associates with Polycomb Repressive Complex 2 and Regulates Mammary Epithelial Differentiation. PLoS Genet. 2012;8:1–20.
Haemmerle M, Gutschner T. Long non-coding RNAs in cancer and development: where do we go from here? Int J Mol Sci. 2015;16:1395–405.
Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70.
Spizzo R, Almeida MI, Colombatti A, Calin GA. Long non-coding RNAs and cancer: a new frontier of translational research? Oncogene. 2012;31:4577–87.
Gibb EA, Vucic EA, Enfield KSS, Stewart GL, Lonergan KM, Kennett JY, et al. Human cancer long non-coding RNA transcriptomes. PLoS One. 2011;6, e25915.
Gutschner T, Diederichs S. The hallmarks of cancer: a long non-coding RNA point of view. RNA Biol. 2012;9:703–19.
Huarte M, Rinn JL. Large non-coding RNAs: missing links in cancer? Hum Mol Genet. 2010;19:R152–61.
Prensner JR, Chinnaiyan AM. The emergence of lncRNAs in cancer biology. Cancer Discov. 2011;1:391–407.
Gibb EA, Brown CJ, Lam WL. The functional role of long non-coding RNA in human carcinomas. Mol Cancer. 2011;10:38.
Nie L, Wu H-J, Hsu J-M, Chang S-S, Labaff AM, Li C-W, et al. Long non-coding RNAs: versatile master regulators of gene expression and crucial players in cancer. Am J Transl Res. 2012;4:127–50.
Cheetham SW, Gruhl F, Mattick JS, Dinger ME. Long noncoding RNAs and the genetics of cancer. Br J Cancer. 2013;108:2419–25.
Qiu M-T, Hu J-W, Yin R, Xu L. Long noncoding RNA: an emerging paradigm of cancer research. Tumour Biol. 2013;34:613–20.
Qi P, Du X. The long non-coding RNAs, a new cancer diagnostic and therapeutic gold mine. Mod Pathol. 2013;26:155–65.
Brannan CI, Dees EC, Ingram RS, Tilghman SM. The product of the H19 gene may function as an RNA. Mol Cell Biol. 1990;10:28–36.
Rachmilewitz J, Gileadi O, Eldar-Geva T, Schneider T, de-Groot N, Hochberg A. Transcription of the H19 gene in differentiating cytotrophoblasts from human placenta. Mol Reprod Dev. 1992;32:196–202.
Jinno Y, Ikeda Y, Yun K, Maw M, Masuzaki H, Fukuda H, et al. Establishment of functional imprinting of the H19 gene in human developing placentae. Nat Genet. 1995;10:318–24.
Cai X, Cullen BR. The imprinted H19 noncoding RNA is a primary microRNA precursor. RNA. 2007;13:313–6.
Leighton PA, Ingram RS, Eggenschwiler J, Efstratiadis A, Tilghman SM. Disruption of imprinting caused by deletion of the H19 gene region in mice. Nature. 1995;375:34–9.
Dugimont T, Montpellier C, Adriaenssens E, Lottin S, Dumont L, Iotsova V, et al. The H19 TATA-less promoter is efficiently repressed by wild-type tumor suppressor gene product p53. Oncogene. 1998;16:2395–401.
Barsyte-Lovejoy D, Lau SK, Boutros PC, Khosravi F, Jurisica I, Andrulis IL, et al. The c-Myc oncogene directly induces the H19 noncoding RNA by allele-specific binding to potentiate tumorigenesis. Cancer Res. 2006;66:5330–7.
Matouk IJ, DeGroot N, Mezan S, Ayesh S, Abu-lail R, Hochberg A, et al. The H19 non-coding RNA is essential for human tumor growth. PLoS One. 2007;2, e845.
Matouk I, Raveh E, Ohana P, Lail R, Gershtain E, Gilon M, et al. The Increasing Complexity of the Oncofetal H19 Gene Locus: Functional Dissection and Therapeutic Intervention. Int J Mol Sci. 2013;14:4298–316.
Fellig Y, Ariel I, Ohana P, Schachter P, Sinelnikov I, Birman T, et al. H19 expression in hepatic metastases from a range of human carcinomas. J Clin Pathol. 2005;58:1064–8.
Vernucci M, Cerrato F, Besnard N, Casola S, Pedone PV, Bruni CB, et al. The H19 endodermal enhancer is required for Igf2 activation and tumor formation in experimental liver carcinogenesis. Oncogene. 2000;19:6376–85.
Berteaux N, Lottin S, Monté D, Pinte S, Quatannens B, Coll J, et al. H19 mRNA-like noncoding RNA promotes breast cancer cell proliferation through positive control by E2F1. J Biol Chem. 2005;280:29625–36.
Cui H, Onyango P, Brandenburg S, Wu Y, Hsieh C-L, Feinberg AP. Loss of imprinting in colorectal cancer linked to hypomethylation of H19 and IGF2. Cancer Res. 2002;62:6442–6.
Hibi K, Nakamura H, Hirai A, Fujikake Y, Kasai Y, Akiyama S, et al. Loss of H19 imprinting in esophageal cancer. Cancer Res. 1996;56:480–2.
Matouk IJ, Mezan S, Mizrahi A, Ohana P, Abu-Lail R, Fellig Y, et al. The oncofetal H19 RNA connection: hypoxia, p53 and cancer. Biochim Biophys Acta. 1803;2010:443–51.
Ma C, Nong K, Zhu H, Wang W, Huang X, Yuan Z, et al. H19 promotes pancreatic cancer metastasis by derepressing let-7’s suppression on its target HMGA2-mediated EMT. Tumour Biol. 2014;35:9163–9.
Li H, Yu B, Li J, Su L, Yan M, Zhu Z, et al. Overexpression of lncRNA H19 enhances carcinogenesis and metastasis of gastric cancer. Oncotarget. 2014;5:2318–29.
Luo M, Li Z, Wang W, Zeng Y, Liu Z, Qiu J. Upregulated H19 contributes to bladder cancer cell proliferation by regulating ID2 expression. FEBS J. 2013;280:1709–16.
Douc-Rasy S, Barrois M, Fogel S, Ahomadegbe JC, Stéhelin D, Coll J, et al. High incidence of loss of heterozygosity and abnormal imprinting of H19 and IGF2 genes in invasive cervical carcinomas. Uncoupling of H19 and IGF2 expression and biallelic hypomethylation of H19. Oncogene. 1996;12:423–30.
Matouk I, Ohana P, Ayesh S, Sidi A, Czerniak A, de Groot N, et al. The Oncofetal H19 RNA in human cancer, from the bench to the patient Review Article. Cancer Ther. 2005;3:249–66.
Ariel I, Miao HQ, Ji XR, Schneider T, Roll D, de Groot N, et al. Imprinted H19 oncofetal RNA is a candidate tumour marker for hepatocellular carcinoma. Mol Pathol. 1998;51:21–5.
Keniry A, Oxley D, Monnier P, Kyba M, Dandolo L, Smits G, et al. The H19 lincRNA is a developmental reservoir of miR-675 that suppresses growth and Igf1r. Nat Cell Biol. 2012;14:659–65.
Mohammad F, Pandey RR, Nagano T, Chakalova L, Mondal T, Fraser P, et al. Kcnq1ot1/Lit1 noncoding RNA mediates transcriptional silencing by targeting to the perinucleolar region. Mol Cell Biol. 2008;28:3713–28.
Nakano S, Murakami K, Meguro M, Soejima H, Higashimoto K, Urano T, et al. Expression profile of LIT1/KCNQ1OT1 and epigenetic status at the KvDMR1 in colorectal cancers. Cancer Sci. 2006;97:1147–54.
Pandey RR, Mondal T, Mohammad F, Enroth S, Redrup L, Komorowski J, et al. Kcnq1ot1 antisense noncoding RNA mediates lineage-specific transcriptional silencing through chromatin-level regulation. Mol Cell. 2008;32:232–46.
Wagschal A, Sutherland HG, Woodfine K, Henckel A, Chebli K, Schulz R, et al. G9a histone methyltransferase contributes to imprinting in the mouse placenta. Mol Cell Biol. 2008;28:1104–13.
Weksberg R, Shuman C, Caluseriu O, Smith AC, Fei Y-L, Nishikawa J, et al. Discordant KCNQ1OT1 imprinting in sets of monozygotic twins discordant for Beckwith-Wiedemann syndrome. Hum Mol Genet. 2002;11:1317–25.
Higashimoto K, Soejima H, Saito T, Okumura K, Mukai T. Imprinting disruption of the CDKN1C/KCNQ1OT1 domain: the molecular mechanisms causing Beckwith-Wiedemann syndrome and cancer. Cytogenet Genome Res. 2006;113:306–12.
Wan J, Huang M, Zhao H, Wang C, Zhao X, Jiang X, et al. A novel tetranucleotide repeat polymorphism within KCNQ1OT1 confers risk for hepatocellular carcinoma. DNA Cell Biol. 2013;32:628–34.
Wijnen M, Alders M, Zwaan CM, Wagner A, van den Heuvel-Eibrink MM. KCNQ1OT1 hypomethylation: a novel disguised genetic predisposition in sporadic pediatric adrenocortical tumors? Pediatr Blood Cancer. 2012;59:565–6.
Yu W, Gius D, Onyango P, Muldoon-Jacobs K, Karp J, Feinberg AP, et al. Epigenetic silencing of tumour suppressor gene p15 by its antisense RNA. Nature. 2008;451:202–6.
Folkersen L, Kyriakou T, Goel A, Peden J, Mälarstig A, Paulsson-Berne G, et al. Relationship between CAD risk genotype in the chromosome 9p21 locus and gene expression. Identification of eight new ANRIL splice variants. PLoS One. 2009;4:e7677.
Popov N, Gil J. Epigenetic regulation of the INK4b-ARF-INK4a locus: in sickness and in health. Epigenetics. 2010;5:685–90.
Pasmant E, Sabbagh A, Vidaud M, Bièche I. ANRIL, a long, noncoding RNA, is an unexpected major hotspot in GWAS. FASEB J. 2011;25:444–8.
Iacobucci I, Sazzini M, Garagnani P, Ferrari A, Boattini A, Lonetti A, et al. A polymorphism in the chromosome 9p21 ANRIL locus is associated to Philadelphia positive acute lymphoblastic leukemia. Leuk Res. 2011;35:1052–9.
Brown CJ, Ballabio A, Rupert JL, Lafreniere RG, Grompe M, Tonlorenzi R, et al. A gene from the region of the human X inactivation centre is expressed exclusively from the inactive X chromosome. Nature. 1991;349:38–44.
Payer B, Lee JT. X chromosome dosage compensation: how mammals keep the balance. Annu Rev Genet. 2008;42:733–72.
Kanduri C, Whitehead J, Mohammad F. The long and the short of it: RNA-directed chromatin asymmetry in mammalian X-chromosome inactivation. FEBS Lett. 2009;583:857–64.
Leeb M, Steffen PA, Wutz A. X chromosome inactivation sparked by non-coding RNAs. RNA Biol. 2009;6:94–9.
Huang K-C, Rao PH, Lau CC, Heard E, Ng S-K, Brown C, et al. Relationship of XIST expression and responses of ovarian cancer to chemotherapy. Mol Cancer Ther. 2002;1:769–76.
Benoît M-H, Hudson TJ, Maire G, Squire JA, Arcand SL, Provencher D, et al. Global analysis of chromosome X gene expression in primary cultures of normal ovarian surface epithelial cells and epithelial ovarian cancer cell lines. Int J Oncol. 2007;30:5–17.
Kawakami T, Zhang C, Taniguchi T, Kim CJ, Okada Y, Sugihara H, et al. Characterization of loss-of-inactive X in Klinefelter syndrome and female-derived cancer cells. Oncogene. 2004;23:6163–9.
Pageau GJ, Hall LL, Ganesan S, Livingston DM, Lawrence JB. The disappearing Barr body in breast and ovarian cancers. Nat Rev Cancer. 2007;7:628–33.
Ayoub N, Richler C, Wahrman J. Xist RNA is associated with the transcriptionally inactive XY body in mammalian male meiosis. Chromosoma. 1997;106:1–10.
McCarrey JR, Watson C, Atencio J, Ostermeier GC, Marahrens Y, Jaenisch R, et al. X-chromosome inactivation during spermatogenesis is regulated by an Xist/Tsix-independent mechanism in the mouse. Genesis. 2002;34:257–66.
Looijenga LH, Gillis AJ, van Gurp RJ, Verkerk AJ, Oosterhuis JW. X inactivation in human testicular tumors. XIST expression and androgen receptor methylation status. Am J Pathol. 1997;151:581–90.
Kawakami T, Okamoto K, Sugihara H, Hattori T, Reeve AE, Ogawa O, et al. The roles of supernumerical X chromosomes and XIST expression in testicular germ cell tumors. J Urol. 2003;169:1546–52.
Kawakami T, Okamoto K, Ogawa O, Okada Y. XIST unmethylated DNA fragments in male-derived plasma as a tumour marker for testicular cancer. Lancet. 2004;363:40–2.
Kleinheinz A, Schulze W. Klinefelter’s syndrome: new and rapid diagnosis by PCR analysis of XIST gene expression. Andrologia. 1994;26:127–9.
Teixeira MR, Pandis N, Dietrich CU, Reed W, Andersen J, Qvist H, et al. Chromosome banding analysis of gynecomastias and breast carcinomas in men. Genes Chromosomes Cancer. 1998;23:16–20.
Rudas M, Schmidinger M, Wenzel C, Okamoto I, Budinsky A, Fazeny B, et al. Karyotypic findings in two cases of male breast cancer. Cancer Genet Cytogenet. 2000;121:190–3.
Rack KA, Chelly J, Gibbons RJ, Rider S, Benjamin D, Lafreniére RG, et al. Absence of the XIST gene from late-replicating isodicentric X chromosomes in leukaemia. Hum Mol Genet. 1994;3:1053–9.
McDonald HL, Gascoyne RD, Horsman D, Brown CJ. Involvement of the X chromosome in non-Hodgkin lymphoma. Genes Chromosomes Cancer. 2000;28:246–57.
Weakley SM, Wang H, Yao Q, Chen C. Expression and function of a large non-coding RNA gene XIST in human cancer. World J Surg. 2011;35:1751–6.
Croce CM. LINCing chromatin remodeling to metastasis. Nat Biotechnol. 2010;28:931–2.
Gupta RA, Shah N, Wang KC, Kim J, Horlings HM, Wong DJ, et al. Long non-coding RNA HOTAIR reprograms chromatin state to promote cancer metastasis. Nature. 2010;464:1071–6.
Kogo R, Shimamura T, Mimori K, Kawahara K, Imoto S, Sudo T, et al. Long noncoding RNA HOTAIR regulates polycomb-dependent chromatin modification and is associated with poor prognosis in colorectal cancers. Cancer Res. 2011;71:6320–6.
Lu L, Zhu G, Zhang C, Deng Q, Katsaros D, Mayne ST, et al. Association of large noncoding RNA HOTAIR expression and its downstream intergenic CpG island methylation with survival in breast cancer. Breast Cancer Res Treat. 2012;136:875–83.
Chisholm KM, Wan Y, Li R, Montgomery KD, Chang HY, West RB. Detection of long non-coding RNA in archival tissue: correlation with polycomb protein expression in primary and metastatic breast carcinoma. PLoS One. 2012;7:e47998.
Hansji H, Leung EY, Baguley BC, Finlay GJ, Askarian-Amiri ME. Keeping abreast with long non-coding RNAs in mammary gland development and breast cancer. Front Genet. 2014;5:379.
Geng YJ, Xie SL, Li Q, Ma J, Wang GY. Large intervening non-coding RNA HOTAIR is associated with hepatocellular carcinoma progression. J Int Med Res. 2011;39:2119–28.
Yang Z, Zhou L, Wu L-M, Lai M-C, Xie H-Y, Zhang F, et al. Overexpression of long non-coding RNA HOTAIR predicts tumor recurrence in hepatocellular carcinoma patients following liver transplantation. Ann Surg Oncol. 2011;18:1243–50.
Ishibashi M, Kogo R, Shibata K, Sawada G, Takahashi Y, Kurashige J, et al. Clinical significance of the expression of long non-coding RNA HOTAIR in primary hepatocellular carcinoma. Oncol Rep. 2013;29:946–50.
Niinuma T, Suzuki H, Nojima M, Nosho K, Yamamoto H, Takamaru H, et al. Upregulation of miR-196a and HOTAIR drive malignant character in gastrointestinal stromal tumors. Cancer Res. 2012;72:1126–36.
Hajjari M, Behmanesh M, Sadeghizadeh M, Zeinoddini M. Up-regulation of HOTAIR long non-coding RNA in human gastric adenocarcinoma tissues. Med Oncol. 2013;30:670.
Liu X, Liu Z, Sun M, Liu J, Wang Z, De W. The long non-coding RNA HOTAIR indicates a poor prognosis and promotes metastasis in non-small cell lung cancer. BMC Cancer. 2013;13:464.
Sunwoo H, Dinger ME, Wilusz JE, Amaral PP, Mattick JS, Spector DL. MEN epsilon/beta nuclear-retained non-coding RNAs are up-regulated upon muscle differentiation and are essential components of paraspeckles. Genome Res. 2009;19:347–59.
Naganuma T, Hirose T. Paraspeckle formation during the biogenesis of long non-coding RNAs. RNA Biol. 2013;10:456–61.
Zeng C, Xu Y, Xu L, Yu X, Cheng J, Yang L, et al. Inhibition of long non-coding RNA NEAT1 impairs myeloid differentiation in acute promyelocytic leukemia cells. BMC Cancer. 2014;14:693.
Halford C. Preliminary investigation of the effects of silencing the non-coding RNA, NEAT1, on the Burkitt’s lymphoma cell line BJAB. Biosci Horizons. 2013;6:hzt006.
Ji P, Diederichs S, Wang W, Böing S, Metzger R, Schneider PM, et al. MALAT-1, a novel noncoding RNA, and thymosin beta4 predict metastasis and survival in early-stage non-small cell lung cancer. Oncogene. 2003;22:8031–41.
Tano K, Mizuno R, Okada T, Rakwal R, Shibato J, Masuo Y, et al. MALAT-1 enhances cell motility of lung adenocarcinoma cells by influencing the expression of motility-related genes. FEBS Lett. 2010;584:4575–80.
Lin R, Maeda S, Liu C, Karin M, Edgington TS. A large noncoding RNA is a marker for murine hepatocellular carcinomas and a spectrum of human carcinomas. Oncogene. 2007;26:851–8.
Lai M, Yang Z, Zhou L, Zhu Q, Xie H, Zhang F, et al. Long non-coding RNA MALAT-1 overexpression predicts tumor recurrence of hepatocellular carcinoma after liver transplantation. Med Oncol. 2012;29:1810–6.
Ying L, Chen Q, Wang Y, Zhou Z, Huang Y, Qiu F. Upregulated MALAT-1 contributes to bladder cancer cell migration by inducing epithelial-to-mesenchymal transition. Mol Biosyst. 2012;8:2289–94.
Han Y, Liu Y, Nie L, Gui Y, Cai Z. Inducing cell proliferation inhibition, apoptosis, and motility reduction by silencing long noncoding ribonucleic acid metastasis-associated lung adenocarcinoma transcript 1 in urothelial carcinoma of the bladder. Urology. 2013;81:209. e1–7.
Liu J-H, Chen G, Dang Y-W, Li C-J, Luo D-Z. Expression and prognostic significance of lncRNA MALAT1 in pancreatic cancer tissues. Asian Pac J Cancer Prev. 2014;15:2971–7.
Guo F, Li Y, Liu Y, Wang J, Li Y, Li G. Inhibition of metastasis-associated lung adenocarcinoma transcript 1 in CaSki human cervical cancer cells suppresses cell proliferation and invasion. Acta Biochim Biophys Sin (Shanghai). 2010;42:224–9.
Guffanti A, Iacono M, Pelucchi P, Kim N, Soldà G, Croft LJ, et al. A transcriptional sketch of a primary human breast cancer by 454 deep sequencing. BMC Genomics. 2009;10:163.
Ren S, Liu Y, Xu W, Sun Y, Lu J, Wang F, et al. Long noncoding RNA MALAT-1 is a new potential therapeutic target for castration resistant prostate cancer. J Urol. 2013;190:2278–87.
Ji Q, Zhang L, Liu X, Zhou L, Wang W, Han Z, et al. Long non-coding RNA MALAT1 promotes tumour growth and metastasis in colorectal cancer through binding to SFPQ and releasing oncogene PTBP2 from SFPQ/PTBP2 complex. Br J Cancer. 2014;111:736–48.
Yamada K, Kano J, Tsunoda H, Yoshikawa H, Okubo C, Ishiyama T, et al. Phenotypic characterization of endometrial stromal sarcoma of the uterus. Cancer Sci. 2006;97:106–12.
Gutschner T, Hämmerle M, Eissmann M, Hsu J, Kim Y, Hung G, et al. The noncoding RNA MALAT1 is a critical regulator of the metastasis phenotype of lung cancer cells. Cancer Res. 2013;73:1180–9.
Zhang B, Arun G, Mao YS, Lazar Z, Hung G, Bhattacharjee G, et al. The lncRNA Malat1 is dispensable for mouse development but its transcription plays a cis-regulatory role in the adult. Cell Rep. 2012;2:111–23.
Kawashima H, Takano H, Sugita S, Takahara Y, Sugimura K, Nakatani T. A novel steroid receptor co-activator protein (SRAP) as an alternative form of steroid receptor RNA-activator gene: expression in prostate cancer cells and enhancement of androgen receptor activity. Biochem J. 2003;369(Pt 1):163–71.
Hube F, Guo J, Chooniedass-Kothari S, Cooper C, Hamedani MK, Dibrov AA, et al. Alternative splicing of the first intron of the steroid receptor RNA activator (SRA) participates in the generation of coding and noncoding RNA isoforms in breast cancer cell lines. DNA Cell Biol. 2006;25:418–28.
Cooper C, Vincett D, Yan Y, Hamedani MK, Myal Y, Leygue E. Steroid Receptor RNA Activator bi-faceted genetic system: Heads or Tails? Biochimie. 2011;93:1973–80.
Leygue E, Dotzlaw H, Watson PH, Murphy LC. Expression of the steroid receptor RNA activator in human breast tumors. Cancer Res. 1999;59:4190–3.
Chooniedass-Kothari S, Hamedani MK, Troup S, Hubé F, Leygue E. The steroid receptor RNA activator protein is expressed in breast tumor tissues. Int J Cancer. 2006;118:1054–9.
Lanz RB, Chua SS, Barron N, Söder BM, DeMayo F, O’Malley BW. Steroid receptor RNA activator stimulates proliferation as well as apoptosis in vivo. Mol Cell Biol. 2003;23:7163–76.
Mourtada-Maarabouni M, Hedge VL, Kirkham L, Farzaneh F, Williams GT. Growth arrest in human T-cells is controlled by the non-coding RNA growth-arrest-specific transcript 5 (GAS5). J Cell Sci. 2008;121(Pt 7):939–46.
Pickard MR, Williams GT. Regulation of apoptosis by long non-coding RNA GAS5 in breast cancer cells: implications for chemotherapy. Breast Cancer Res Treat. 2014;145:359–70.
Mourtada-Maarabouni M, Hasan AM, Farzaneh F, Williams GT. Inhibition of human T-cell proliferation by mammalian target of rapamycin (mTOR) antagonists requires noncoding RNA growth-arrest-specific transcript 5 (GAS5). Mol Pharmacol. 2010;78:19–28.
Zhang Z, Zhu Z, Watabe K, Zhang X, Bai C, Xu M, et al. Negative regulation of lncRNA GAS5 by miR-21. Cell Death Differ. 2013;20:1558–68.
Mourtada-Maarabouni M, Pickard MR, Hedge VL, Farzaneh F, Williams GT. GAS5, a non-protein-coding RNA, controls apoptosis and is downregulated in breast cancer. Oncogene. 2009;28:195–208.
Pickard MR, Mourtada-Maarabouni M, Williams GT. Long non-coding RNA GAS5 regulates apoptosis in prostate cancer cell lines. Biochim Biophys Acta. 1832;2013:1613–23.
Sun M, Jin F, Xia R, Kong R, Li J, Xu T, et al. Decreased expression of long noncoding RNA GAS5 indicates a poor prognosis and promotes cell proliferation in gastric cancer. BMC Cancer. 2014;14:319.
Cao S, Liu W, Li F, Zhao W, Qin C. Decreased expression of lncRNA GAS5 predicts a poor prognosis in cervical cancer. Int J Clin Exp Pathol. 2014;7:6776–83.
Qiao H-P, Gao W-S, Huo J-X, Yang Z-S. Long Non-coding RNA GAS5 Functions as a Tumor Suppressor in Renal Cell Carcinoma. Asian Pacific J Cancer Prev. 2013;14:1077–82.
Liu Z, Wang W, Jiang J, Bao E, Xu D, Zeng Y, et al. Downregulation of GAS5 promotes bladder cancer cell proliferation, partly by regulating CDK6. PLoS One. 2013;8:e73991.
Meyne J, Ratliff RL, Moyzis RK. Conservation of the human telomere sequence (TTAGGG)n among vertebrates. Proc Natl Acad Sci U S A. 1989;86:7049–53.
Counter CM, Avilion AA, LeFeuvre CE, Stewart NG, Greider CW, Harley CB, et al. Telomere shortening associated with chromosome instability is arrested in immortal cells which express telomerase activity. EMBO J. 1992;11:1921–9.
Feng J, Funk WD, Wang SS, Weinrich SL, Avilion AA, Chiu CP, et al. The RNA component of human telomerase. Science. 1995;269:1236–41.
Azzalin CM, Reichenbach P, Khoriauli L, Giulotto E, Lingner J. Telomeric repeat containing RNA and RNA surveillance factors at mammalian chromosome ends. Science. 2007;318:798–801.
Redon S, Reichenbach P, Lingner J. The non-coding RNA TERRA is a natural ligand and direct inhibitor of human telomerase. Nucleic Acids Res. 2010;38:5797–806.
Ng LJ, Cropley JE, Pickett HA, Reddel RR, Suter CM. Telomerase activity is associated with an increase in DNA methylation at the proximal subtelomere and a reduction in telomeric transcription. Nucleic Acids Res. 2009;37:1152–9.
Caslini C. Transcriptional regulation of telomeric non-coding RNA: implications on telomere biology, replicative senescence and cancer. RNA Biol. 2010;7:18–22.
Bejerano G, Pheasant M, Makunin I, Stephen S, Kent WJ, Mattick JS, et al. Ultraconserved elements in the human genome. Science. 2004;304:1321–5.
Calin GA, Liu C, Ferracin M, Hyslop T, Spizzo R, Sevignani C, et al. Ultraconserved regions encoding ncRNAs are altered in human leukemias and carcinomas. Cancer Cell. 2007;12:215–29.
Scaruffi P, Stigliani S, Moretti S, Coco S, De Vecchi C, Valdora F, et al. Transcribed-Ultra Conserved Region expression is associated with outcome in high-risk neuroblastoma. BMC Cancer. 2009;9:441.
Mestdagh P, Fredlund E, Pattyn F, Rihani A, Van Maerken T, Vermeulen J, et al. An integrative genomics screen uncovers ncRNA T-UCR functions in neuroblastoma tumours. Oncogene. 2010;29:3583–92.
Braconi C, Valeri N, Kogure T, Gasparini P, Huang N, Nuovo GJ, et al. Expression and functional role of a transcribed noncoding RNA with an ultraconserved element in hepatocellular carcinoma. Proc Natl Acad Sci U S A. 2011;108:786–91.
Hudson RS, Yi M, Volfovsky N, Prueitt RL, Esposito D, Volinia S, et al. Transcription signatures encoded by ultraconserved genomic regions in human prostate cancer. Mol Cancer. 2013;12:13.
Peng JC, Shen J, Ran ZH. Transcribed ultraconserved region in human cancers. RNA Biol. 2013;10:1771–7.
Panzitt K, Tschernatsch MMO, Guelly C, Moustafa T, Stradner M, Strohmaier HM, et al. Characterization of HULC, a novel gene with striking up-regulation in hepatocellular carcinoma, as noncoding RNA. Gastroenterology. 2007;132:330–42.
Liu Y, Pan S, Liu L, Zhai X, Liu J, Wen J, et al. A genetic variant in long non-coding RNA HULC contributes to risk of HBV-related hepatocellular carcinoma in a Chinese population. PLoS One. 2012;7:e35145.
Wang J, Liu X, Wu H, Ni P, Gu Z, Qiao Y, et al. CREB up-regulates long non-coding RNA, HULC expression through interaction with microRNA-372 in liver cancer. Nucleic Acids Res. 2010;38:5366–83.
Xie H, Ma H, Zhou D. Plasma HULC as a promising novel biomarker for the detection of hepatocellular carcinoma. Biomed Res Int. 2013;2013:136106.
Matouk IJ, Abbasi I, Hochberg A, Galun E, Dweik H, Akkawi M. Highly upregulated in liver cancer noncoding RNA is overexpressed in hepatic colorectal metastasis. Eur J Gastroenterol Hepatol. 2009;21:688–92.
Yang F, Zhang L, Huo X, Yuan J, Xu D, Yuan S, et al. Long noncoding RNA high expression in hepatocellular carcinoma facilitates tumor growth through enhancer of zeste homolog 2 in humans. Hepatology. 2011;54:1679–89.
Tinzl M, Marberger M, Horvath S, Chypre C. DD3PCA3 RNA analysis in urine–a new perspective for detecting prostate cancer. Eur Urol. 2004;46:182–6. discussion 187.
Hessels D, Schalken JA. The use of PCA3 in the diagnosis of prostate cancer. Nat Rev Urol. 2009;6:255–61.
Srikantan V, Zou Z, Petrovics G, Xu L, Augustus M, Davis L, et al. PCGEM1, a prostate-specific gene, is overexpressed in prostate cancer. Proc Natl Acad Sci U S A. 2000;97:12216–21.
Petrovics G, Zhang W, Makarem M, Street JP, Connelly R, Sun L, et al. Elevated expression of PCGEM1, a prostate-specific gene with cell growth-promoting function, is associated with high-risk prostate cancer patients. Oncogene. 2004;23:605–11.
Prensner JR, Iyer MK, Balbin OA, Dhanasekaran SM, Cao Q, Brenner JC, et al. Transcriptome sequencing across a prostate cancer cohort identifies PCAT-1, an unannotated lincRNA implicated in disease progression. Nat Biotechnol. 2011;29:742–9.
Prensner JR, Chen W, Iyer MK, Cao Q, Ma T, Han S, et al. PCAT-1, a long noncoding RNA, regulates BRCA2 and controls homologous recombination in cancer. Cancer Res. 2014;74:1651–60.
Pickl JMA, Heckmann D, Ratz L, Klauck SM, Sültmann H. Novel RNA markers in prostate cancer: functional considerations and clinical translation. Biomed Res Int. 2014;2014:765207.
Korneev SA, Korneeva EI, Lagarkova MA, Kiselev SL, Critchley G, O’Shea M. Novel noncoding antisense RNA transcribed from human anti-NOS2A locus is differentially regulated during neuronal differentiation of embryonic stem cells. RNA. 2008;14:2030–7.
Zhang X, Lian Z, Padden C, Gerstein MB, Rozowsky J, Snyder M, et al. A myelopoiesis-associated regulatory intergenic noncoding RNA transcript within the human HOXA cluster. Blood. 2009;113:2526–34.
Garding A, Bhattacharya N, Claus R, Ruppel M, Tschuch C, Filarsky K, et al. Epigenetic upregulation of lncRNAs at 13q14.3 in leukemia is linked to the In Cis downregulation of a gene cluster that targets NF-kB. PLoS Genet. 2013;9:e1003373.
Stilgenbauer S, Nickolenko J, Wilhelm J, Wolf S, Weitz S, Döhner K, et al. Expressed sequences as candidates for a novel tumor suppressor gene at band 13q14 in B-cell chronic lymphocytic leukemia and mantle cell lymphoma. Oncogene. 1998;16:1891–7.
Ouillette P, Erba H, Kujawski L, Kaminski M, Shedden K, Malek SN. Integrated genomic profiling of chronic lymphocytic leukemia identifies subtypes of deletion 13q14. Cancer Res. 2008;68:1012–21.
Lerner M, Harada M, Lovén J, Castro J, Davis Z, Oscier D, et al. DLEU2, frequently deleted in malignancy, functions as a critical host gene of the cell cycle inhibitory microRNAs miR-15a and miR-16-1. Exp Cell Res. 2009;315:2941–52.
Klein U, Lia M, Crespo M, Siegel R, Shen Q, Mo T, et al. The DLEU2/miR-15a/16-1 cluster controls B cell proliferation and its deletion leads to chronic lymphocytic leukemia. Cancer Cell. 2010;17:28–40.
Liu W-T, Lu X, Tang G-H, Ren J-J, Liao W-J, Ge P-L, et al. LncRNAs expression signatures of hepatocellular carcinoma revealed by microarray. World J Gastroenterol. 2014;20:6314–21.
Zhu J, Liu S, Ye F, Shen Y, Tie Y, Zhu J, et al. The long noncoding RNA expression profile of hepatocellular carcinoma identified by microarray analysis. PLoS One. 2014;9:e101707.
Hughes JM, Salvatori B, Giorgi FM, Bozzoni I, Fatica A. CEBPA-regulated lncRNAs, new players in the study of acute myeloid leukemia. J Hematol Oncol. 2014;7:69.
Fang K, Han B-W, Chen Z-H, Lin K-Y, Zeng C-W, Li X-J, et al. A distinct set of long non-coding RNAs in childhood MLL-rearranged acute lymphoblastic leukemia: biology and epigenetic target. Hum Mol Genet. 2014;23:3278–88.
Reiche K, Kasack K, Schreiber S, Lüders T, Due EU, Naume B, et al. Long non-coding RNAs differentially expressed between normal versus primary breast tumor tissues disclose converse changes to breast cancer-related protein-coding genes. PLoS One. 2014;9:e106076.
Wapinski O, Chang HY. Long noncoding RNAs and human disease. Trends Cell Biol. 2011;21:354–61.
Chen G, Wang Z, Wang D, Qiu C, Liu M, Chen X, et al. LncRNADisease: a database for long-non-coding RNA-associated diseases. Nucleic Acids Res. 2013;41(Database issue):D983–6.
Wahlestedt C. Targeting long non-coding RNA to therapeutically upregulate gene expression. Nat Rev Drug Discov. 2013;12:433–46.
Tong Y-K, Lo YMD. Diagnostic developments involving cell-free (circulating) nucleic acids. Clin Chim Acta. 2006;363:187–96.
Ayers D. Long Non-Coding RNAs: Novel Emergent Biomarkers for Cancer Diagnostics. J Cancer Res Treat. 2013;1:31–5.
Sánchez Y, Huarte M. Long non-coding RNAs: challenges for diagnosis and therapies. Nucleic Acid Ther. 2013;23:15–20.
Fatemi RP, Velmeshev D, Faghihi MA. De-repressing LncRNA-Targeted Genes to Upregulate Gene Expression: Focus on Small Molecule Therapeutics. Mol Ther Nucleic Acids. 2014;3, e196.
Takahashi H, Carninci P. Widespread genome transcription: new possibilities for RNA therapies. Biochem Biophys Res Commun. 2014;452:294–301.
Yao Y, Li J, Wang L. Large Intervening Non-Coding RNA HOTAIR Is an Indicator of Poor Prognosis and a Therapeutic Target in Human Cancers. Int J Mol Sci. 2014;15:18985–99.
Bhan A, Hussain I, Ansari KI, Kasiri S, Bashyal A, Mandal SS. Antisense transcript long noncoding RNA (lncRNA) HOTAIR is transcriptionally induced by estradiol. J Mol Biol. 2013;425:3707–22.
Kim K, Jutooru I, Chadalapaka G, Johnson G, Frank J, Burghardt R, et al. HOTAIR is a negative prognostic factor and exhibits pro-oncogenic activity in pancreatic cancer. Oncogene. 2013;32:1616–25.
Ferreira LB, Palumbo A, de Mello KD, Sternberg C, Caetano MS, de Oliveira FL, et al. PCA3 noncoding RNA is involved in the control of prostate-cancer cell survival and modulates androgen receptor signaling. BMC Cancer. 2012;12:507.
Yang L, Lin C, Jin C, Yang JC, Tanasa B, Li W, et al. lncRNA-dependent mechanisms of androgen-receptor-regulated gene activation programs. Nature. 2013;500:598–602.
Crea F, Watahiki A, Quagliata L, Xue H, Pikor L, Parolia A, et al. Identification of a long non-coding RNA as a novel biomarker and potential therapeutic target for metastatic prostate cancer. Oncotarget. 2014;5.
Cui Z, Ren S, Lu J, Wang F, Xu W, Sun Y, et al. The prostate cancer-up-regulated long noncoding RNA PlncRNA-1 modulates apoptosis and proliferation through reciprocal regulation of androgen receptor. Urologic oncology. 2013;1117–23.
Yuan S-X, Yang F, Yang Y, Tao Q-F, Zhang J, Huang G, et al. Long noncoding RNA associated with microvascular invasion in hepatocellular carcinoma promotes angiogenesis and serves as a predictor for hepatocellular carcinoma patients’ poor recurrence-free survival after hepatectomy. Hepatology. 2012;56:2231–41.
Tsang WP, Wong TWL, Cheung AHH, Co CNN, Kwok TT. Induction of drug resistance and transformation in human cancer cells by the noncoding RNA CUDR. RNA. 2007;13:890–8.
Wang Y, Chen W, Yang C, Wu W, Wu S, Qin X, et al. Long non-coding RNA UCA1a(CUDR) promotes proliferation and tumorigenesis of bladder cancer. Int J Oncol. 2012;41:276–84.
Thrash-Bingham CA, Tartof KD. aHIF: a Natural Antisense Transcript Overexpressed in Human Renal Cancer and During Hypoxia. JNCI J Natl Cancer Inst. 1999;91:143–51.
Bertozzi D, Iurlaro R, Sordet O, Marinello J, Zaffaroni N, Capranico G. Characterization of novel antisense HIF-1α transcripts in human cancers. Cell Cycle. 2011;10:3189–97.
Ariel I, Sughayer M, Fellig Y, Pizov G, Ayesh S, Podeh D, et al. The imprinted H19 gene is a marker of early recurrence in human bladder carcinoma. Mol Pathol. 2000;53:320–3.
Dorsett Y, Tuschl T. siRNAs: applications in functional genomics and potential as therapeutics. Nat Rev Drug Discov. 2004;3:318–29.
Burnett JC, Rossi JJ, Tiemann K. Current progress of siRNA/shRNA therapeutics in clinical trials. Biotechnol J. 2011;6:1130–46.
Bennett CF, Swayze EE. RNA targeting therapeutics: molecular mechanisms of antisense oligonucleotides as a therapeutic platform. Annu Rev Pharmacol Toxicol. 2010;50:259–93.
Watts JK, Corey DR. Gene silencing by siRNAs and antisense oligonucleotides in the laboratory and the clinic. J Pathol. 2012;226:365–79.
Grillone LR, Lanz R. Fomivirsen. Drugs Today (Barc). 2001;37:245–55.
Wong E, Goldberg T. Mipomersen (kynamro): a novel antisense oligonucleotide inhibitor for the management of homozygous familial hypercholesterolemia. P T. 2014;39:119–22.
Modarresi F, Faghihi MA, Lopez-Toledano MA, Fatemi RP, Magistri M, Brothers SP, et al. Inhibition of natural antisense transcripts in vivo results in gene-specific transcriptional upregulation. Nat Biotechnol. 2012;30:453–9.
Veedu RN, Wengel J. Locked nucleic acids: promising nucleic acid analogs for therapeutic applications. Chem Biodivers. 2010;7:536–42.
Tsai M-C, Spitale RC, Chang HY. Long intergenic noncoding RNAs: new links in cancer progression. Cancer Res. 2011;71:3–7.
Colley SM, Leedman PJ. SRA and its binding partners: an expanding role for RNA-binding coregulators in nuclear receptor-mediated gene regulation. Crit Rev Biochem Mol Biol. 2009;44:25–33.
Mizrahi A, Czerniak A, Levy T, Amiur S, Gallula J, Matouk I, et al. Development of targeted therapy for ovarian cancer mediated by a plasmid expressing diphtheria toxin under the control of H19 regulatory sequences. J Transl Med. 2009;7:69.
Fan M, Li X, Jiang W, Huang Y, Li J, Wang Z. A long non-coding RNA, PTCSC3, as a tumor suppressor and a target of miRNAs in thyroid cancer cells. Exp Ther Med. 2013;5:1143–6.
Poliseno L, Salmena L, Zhang J, Carver B, Haveman WJ, Pandolfi PP. A coding-independent function of gene and pseudogene mRNAs regulates tumour biology. Nature. 2010;465:1033–8.
Zhang X, Gejman R, Mahta A, Zhong Y, Rice KA, Zhou Y, et al. Maternally expressed gene 3, an imprinted noncoding RNA gene, is associated with meningioma pathogenesis and progression. Cancer Res. 2010;70:2350–8.
Wang G, Li Z, Zhao Q, Zhu Y, Zhao C, Li X, et al. LincRNA-p21 enhances the sensitivity of radiotherapy for human colorectal cancer by targeting the Wnt/β-catenin signaling pathway. Oncol Rep. 2014;31:1839–45.
DeVos SL, Miller TM. Antisense oligonucleotides: treating neurodegeneration at the level of RNA. Neurotherapeutics. 2013;10:486–97.
Costa FF. Non-coding RNAs and new opportunities for the private sector. Drug Discov Today. 2009;14:446–52.
Chen W, Böcker W, Brosius J, Tiedge H. Expression of neural BC200 RNA in human tumours. J Pathol. 1997;183:345–51.
Graham LD, Pedersen SK, Brown GS, Ho T, Kassir Z, Moynihan AT, et al. Colorectal Neoplasia Differentially Expressed (CRNDE), a Novel Gene with Elevated Expression in Colorectal Adenomas and Adenocarcinomas. Genes Cancer. 2011;2:829–40.
Ellis BC, Molloy PL, Graham LD. CRNDE: A Long Non-Coding RNA Involved in CanceR, Neurobiology, and Development. Front Genet. 2012;3:270.
Yu M, Ohira M, Li Y, Niizuma H, Oo ML, Zhu Y, et al. High expression of ncRAN, a novel non-coding RNA mapped to chromosome 17q25.1, is associated with poor prognosis in neuroblastoma. Int J Oncol. 2009;34:931–8.
Zhu Y, Yu M, Li Z, Kong C, Bi J, Li J, et al. ncRAN, a newly identified long noncoding RNA, enhances human bladder tumor growth, invasion, and survival. Urology. 2011;77:510. e1–5.
Chung S, Nakagawa H, Uemura M, Piao L, Ashikawa K, Hosono N, et al. Association of a novel long non-coding RNA in 8q24 with prostate cancer susceptibility. Cancer Sci. 2011;102:245–52.
Graham M, Adams JM. Chromosome 8 breakpoint far 3’ of the c-myc oncogene in a Burkitt's lymphoma 2;8 variant translocation is equivalent to the murine pvt-1 locus. EMBO J. 1986;5:2845–51.
Mengle-Gaw L, Rabbitts TH. A human chromosome 8 region with abnormalities in B cell, HTLV-I+ T cell and c-myc amplified tumours. EMBO J. 1987;6:1959–65.
Barsotti AM, Beckerman R, Laptenko O, Huppi K, Caplen NJ, Prives C. p53-Dependent induction of PVT1 and miR-1204. J Biol Chem. 2012;287:2509–19.
Guan Y, Kuo W-L, Stilwell JL, Takano H, Lapuk AV, Fridlyand J, et al. Amplification of PVT1 contributes to the pathophysiology of ovarian and breast cancer. Clin Cancer Res. 2007;13:5745–55.
Yang Y-R, Zang S-Z, Zhong C-L, Li Y-X, Zhao S-S, Feng X-J. Increased expression of the lncRNA PVT1 promotes tumorigenesis in non-small cell lung cancer. Int J Clin Exp Pathol. 2014;7:6929–35.
Wang F, Yuan J-H, Wang S-B, Yang F, Yuan S-X, Ye C, et al. Oncofetal long noncoding RNA PVT1 promotes proliferation and stem cell-like property of hepatocellular carcinoma cells by stabilizing NOP2. Hepatology. 2014;60:1278–90.
Takahashi Y, Sawada G, Kurashige J, Uchi R, Matsumura T, Ueo H, et al. Amplification of PVT-1 is involved in poor prognosis via apoptosis inhibition in colorectal cancers. Br J Cancer. 2014;110:164–71.
Prensner JR, Iyer MK, Sahu A, Asangani IA, Cao Q, Patel L, et al. The long noncoding RNA SChLAP1 promotes aggressive prostate cancer and antagonizes the SWI/SNF complex. Nat Genet. 2013;45:1392–8.
Han Y, Liu Y, Gui Y, Cai Z. Long intergenic non-coding RNA TUG1 is overexpressed in urothelial carcinoma of the bladder. J Surg Oncol. 2013;107:555–9.
Zhang E, Yin D, Sun M, Kong R, Liu X, You L, et al. P53-regulated long non-coding RNA TUG1 affects cell proliferation in human non-small cell lung cancer, partly through epigenetically regulating HOXB7 expression. Cell Death Dis. 2014;5, e1243.
Yang Y, Li H, Hou S, Hu B, Liu J, Wang J. The noncoding RNA expression profile and the effect of lncRNA AK126698 on cisplatin resistance in non-small-cell lung cancer cell. PLoS One. 2013;8, e65309.
Yang F, Huo X, Yuan S, Zhang L, Zhou W, Wang F, et al. Repression of the long noncoding RNA-LET by histone deacetylase 3 contributes to hypoxia-mediated metastasis. Mol Cell. 2013;49:1083–96.
Zhou Y, Zhang X, Klibanski A. MEG3 noncoding RNA: a tumor suppressor. J Mol Endocrinol. 2012;48:R45–53.
Ying L, Huang Y, Chen H, Wang Y, Xia L, Chen Y, et al. Downregulated MEG3 activates autophagy and increases cell proliferation in bladder cancer. Mol Biosyst. 2013;9:407–11.
Gejman R, Batista DL, Zhong Y, Zhou Y, Zhang X, Swearingen B, et al. Selective loss of MEG3 expression and intergenic differentially methylated region hypermethylation in the MEG3/DLK1 locus in human clinically nonfunctioning pituitary adenomas. J Clin Endocrinol Metab. 2008;93:4119–25.
Benetatos L, Vartholomatos G, Hatzimichael E. MEG3 imprinted gene contribution in tumorigenesis. Int J Cancer. 2011;129:773–9.
Ling H, Spizzo R, Atlasi Y, Nicoloso M, Shimizu M, Redis RS, et al. CCAT2, a novel noncoding RNA mapping to 8q24, underlies metastatic progression and chromosomal instability in colon cancer. Genome Res. 2013;23:1446–61.
Redis RS, Sieuwerts AM, Look MP, Tudoran O, Ivan C, Spizzo R, et al. CCAT2, a novel long non-coding RNA in breast cancer: expression study and clinical correlations. Oncotarget. 2013;4:1748–62.
Li R, Zhang L, Jia L, Duan Y, Li Y, Bao L, et al. Long non-coding RNA BANCR promotes proliferation in malignant melanoma by regulating MAPK pathway activation. PLoS One. 2014;9, e100893.
Su S, Gao J, Wang T, Wang J, Li H, Wang Z. Long non-coding RNA BANCR regulates growth and metastasis and is associated with poor prognosis in retinoblastoma. Tumour Biol 2015.
Sun M, Liu X-H, Wang K-M, Nie F, Kong R, Yang J, et al. Downregulation of BRAF activated non-coding RNA is associated with poor prognosis for non-small cell lung cancer and promotes metastasis by affecting epithelial-mesenchymal transition. Mol Cancer. 2014;13:68.
Yuan J, Yang F, Wang F, Ma J, Guo Y, Tao Q, et al. A long noncoding RNA activated by TGF-β promotes the invasion-metastasis cascade in hepatocellular carcinoma. Cancer Cell. 2014;25:666–81.
Yuan S-X, Tao Q-F, Wang J, Yang F, Liu L, Wang L-L, et al. Antisense long non-coding RNA PCNA-AS1 promotes tumor growth by regulating proliferating cell nuclear antigen in hepatocellular carcinoma. Cancer Lett. 2014;349:87–94.
Wang X-S, Zhang Z, Wang H-C, Cai J-L, Xu Q-W, Li M-Q, et al. Rapid identification of UCA1 as a very sensitive and specific unique marker for human bladder carcinoma. Clin Cancer Res. 2006;12:4851–8.
Wang F, Li X, Xie X, Zhao L, Chen W. UCA1, a non-protein-coding RNA up-regulated in bladder carcinoma and embryo, influencing cell growth and promoting invasion. FEBS Lett. 2008;582:1919–27.
Yang C, Li X, Wang Y, Zhao L, Chen W. Long non-coding RNA UCA1 regulated cell cycle distribution via CREB through PI3-K dependent pathway in bladder carcinoma cells. Gene. 2012;496:8–16.
Huang J, Zhou N, Watabe K, Lu Z, Wu F, Xu M, et al. Long non-coding RNA UCA1 promotes breast tumor growth by suppression of p27 (Kip1). Cell Death Dis. 2014;5, e1008.
Ma M-Z, Chu B-F, Zhang Y, Weng M-Z, Qin Y-Y, Gong W, et al. Long non-coding RNA CCAT1 promotes gallbladder cancer development via negative modulation of miRNA-218-5p. Cell Death Dis. 2015;6, e1583.
Nissan A, Stojadinovic A, Mitrani-Rosenbaum S, Halle D, Grinbaum R, Roistacher M, et al. Colon cancer associated transcript-1: a novel RNA expressed in malignant and pre-malignant human tissues. Int J Cancer. 2012;130:1598–606.
Qiu M, Xu Y, Yang X, Wang J, Hu J, Xu L, et al. CCAT2 is a lung adenocarcinoma-specific long non-coding RNA and promotes invasion of non-small cell lung cancer. Tumour Biol. 2014;35:5375–80.
Acknowledgement
The research work on long noncoding RNA in Rao’s laboratory is supported by the Department of Biotechnology. M. R. S. is a J. C. Bose Fellow and a SERB Distinguished Fellow of the Department of Science and Technology. R.F. is a postdoctoral Fellow at the Department of Biotechnology. V. S. A and D. P. are Senior Research Fellows of the council of Scientific and Industrial Research.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
MRSR designed and edited the review. RF composed the ‘Regulation of gene expression by lncRNAs’, ‘LncRNAs in cancer, LncRNAs as potential biomarkers and therapeutic targets’ sections of the manuscript and created the table. VJ wrote the introduction, ‘similarities and differences between lncRNAs and mRNA’ sections contributed the figure. DP contributed the ‘LncRNAs in early Mammalian Development’ section. All authors read and approved the final manuscript.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Fatima, R., Akhade, V.S., Pal, D. et al. Long noncoding RNAs in development and cancer: potential biomarkers and therapeutic targets. Mol and Cell Ther 3, 5 (2015). https://doi.org/10.1186/s40591-015-0042-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40591-015-0042-6