-
PDF
- Split View
-
Views
-
Cite
Cite
Makoto Yanagisawa, Robert K Yu, The expression and functions of glycoconjugates in neural stem cells, Glycobiology, Volume 17, Issue 7, July 2007, Pages 57R–74R, https://doi.org/10.1093/glycob/cwm018
Close - Share Icon Share
Abstract
The mammalian central nervous system is organized by a variety of cells such as neurons and glial cells. These cells are generated from a common progenitor, the neural stem cell (NSC). NSCs are defined as undifferentiated neural cells that are characterized by their high proliferative potential while retaining the capacity for self-renewal and multipotency. Glycoconjugates carrying carbohydrate antigens, including glycoproteins, glycolipids, and proteoglycans, are primarily localized on the plasma-membrane surface of cells and serve as excellent biomarkers at various stages of cellular differentiation. Moreover, they also play important functional roles in determining cell fate such as self-renewal, proliferation, and differentiation. In the present review, we discuss the expression pattern and possible functions of glycoconjugates and carbohydrate antigens in NSCs, with an emphasis on stage-specific embryonic antigen-1, human natural killer antigen-1, polysialic acid-neural cell-adhesion molecule, prominin-1, gp130, chondroitin sulfate proteoglycans, heparan sulfate proteoglycans, cystatin C, galectin-1, glycolipids, and Notch.
Neural stem cells and glycoconjugates
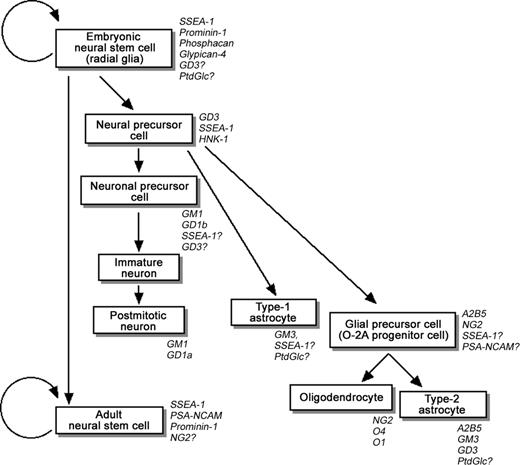
Neural stem cells (NSCs) are defined as undifferentiated neural cells that are endowed with a high potential for proliferation and the capacity for self-renewal; they also can generate a wide variety of differentiated progeny, such as neurons and glial cells (e.g., astrocytes and oligodendrocytes), and retain their multipotency (Figure 1; Temple 1989; Reynolds and Weiss 1992; Reynolds et al. 1992; McKay 1997; Temple and Alvarez-Buylla 1999; Gage 2000; Okano 2002). Because of the biological potential of NSCs in neurogenesis and neural repair, there has been tremendous interest lately in their basic biology and clinical use in treating a variety of neurodegenerative disorders and spinal cord injuries.
A model of the neural-cell lineage derived from NSCs in the developing central nervous system. The glycoconjugate and carbohydrate markers are indicated in italic.
During brain development, NSCs appear in the neuroepithelium and contribute to the major cell types of the early neuroectoderm and the neural tube. Radial glia, bipolar cells transiently appearing in the neuroepithelium, are presumed to play important roles as NSCs at this stage (Malatesta et al. 2000; Hartfuss et al. 2001; Noctor et al. 2001; Götz and Barde 2005). As development proceeds, NSCs are thought to give rise to neurons, astrocytes, and oligodendrocytes (Figure 1). Simultaneously, NSCs become gradually less abundant, and more-restricted neural precursor cells (NPCs) emerge (Note: recently, researchers tend to use the term “NSC” interchangeably with “NPC” without clearly defining the characteristics of the two cell populations. For this reason, in this review we sometimes describe NSCs as “NSCs/NPCs.”). In the adult mammalian brain, NSCs are identified as a kind of glial fibrillary acidic protein (GFAP)-positive astrocytes located primarily in two regions: the subventricular zone (SVZ) of the lateral ventricles and the subgranular layer of the dentate gyrus in the hippocampus (Doetsch et al. 1999; Seri et al. 2001). These adult NSCs are thought to be derived from radial glia (Merkle et al. 2004). Although neurogenesis is mostly completed at birth, interest in NSCs in these two regions arises from the fact that NSCs continue to produce new neurons during adulthood. The NSCs in the SVZ migrate through the rostral migratory pathway to yield neurons in the olfactory bulb. NSCs in the subgranular layer migrate a short distance into the granular cell layer and differentiate into hippocampal granule cells. In addition, it has recently been confirmed that the SVZ NSCs give rise to oligodendrocytes in vivo (Menn et al. 2006). Because of these features, NSCs/NPCs have been considered to represent a cellular reservoir for formation of the central nervous system (CNS) and for replacement of cells lost during normal cellular turnover in adulthood. On the other hand, there is an opposing view that there is a lack of neurogenesis in irradiation-induced adult mouse brain, which does not affect learning ability (Meshi et al. 2006).
The fate of NSCs/NPCs, such as proliferation, differentiation, survival, and death, may be regulated by “niche,” a specialized microenvironment where NSCs are located in vivo (Doetsch 2003; Alvarez-Buylla and Lim 2004). “Niche” is composed of a group of cells in a specialized tissue location serving extrinsic signals and physical anchors by producing soluble factors, membrane-bound molecules and extracellular matrices (ECMs) to maintain stem cells, such as germ line stem cells, hematopoietic stem cells, epithelial stem cells, intestinal stem cells, and NSCs (Li and Xie 2005). In addition, it is also proposed that “niche” has a role not only to maintain stem cells but also to prevent tumorigenesis by controlling the cellular proliferation (Li and Xie 2005). For adult NSCs, endothelial cells forming blood vessels and the basal lamina have an essential role as components of the niche (Doetsch 2003; Shen et al. 2004). The importance of the signal molecules emanating from the NSC niche is well known. For example, basic fibroblast growth factor (bFGF) and epidermal growth factor (EGF) are important in sustaining multipotency and enhancing the proliferation of NSCs/NPCs and in neurogenesis (Reynolds and Weiss 1992; Reynolds et al. 1992; Kuhn et al. 1997; Vaccarino et al. 1999). ECMs including proteoglycans provide reservoirs for growth factors and matrices for cell attachment and migration. Related to this, one of the receptor molecules of the ECM, β1 integrin, has been revealed to mediate signaling for cell adhesion and for self-renewal in NSCs (Campos et al. 2004; Leone et al. 2005). Thus, these studies clearly show the essential roles of niche in the maintenance of the properties of NSCs.
Glycoconjugates refer to biopolymers containing one or more carbohydrate units and are roughly classified into three groups based on their structures: proteoglycans, glycoproteins, and glycolipids. These molecules are ubiquitously distributed in the organs, tissues, cells, and body fluids of animals and plants, and are primarily, although not exclusively, localized on the cell surface. Therefore, glycoconjugates are crucial in determining the properties of cells, participating in signal transduction in response to external stimuli, and in mediating cell–cell interaction and adhesion. Because of the importance of these components during development, glycoconjugates in NSCs/NPCs should be systematically studied for the following reasons. First, glycoconjugates are useful neural cell lineage-specific markers. Because glycoconjugates are localized on the cell surface and the expression patterns are frequently and drastically changed during development (Jessell et al. 1990; Cameron and Rakic 1991; Yu 1994; Zhang 2001; Muramatsu and Muramatsu 2004; Yu and Yanagisawa 2006), they are often utilized as specific biomarkers of cells, including NSCs, at different stages of differentiation (Figure 1). Secondly, recent studies clearly implicate the functional relevance of glycoconjugates in NSCs/NPCs in mediating signal transduction and cell–cell recognition and adhesion. There is also a strong possibility that glycoconjugates, especially proteoglycans that are the major components of the ECM, are involved in mediation of the cell fate-regulating signals generated by the NSC niche. In this review, we will discuss the expression pattern and putative functions of glycoconjugates and related proteins in NSCs/NPCs.
Stage-specific embryonic antigen-1/Lewis x antigen
Stage-specific embryonic antigen-1 (SSEA-1, for review, see Muramatsu and Muramatsu 2004) is the antigen of a monoclonal antibody established by immunization with F9 embryonic carcinoma cells (Solter and Knowles 1978). Studies by Gooi et al. (1981) clearly indicated that the binding reactivity of anti-SSEA-1 antibody to human meconium is inhibited by an oligosaccharide containing the Lewis x structure [Lex; Galβ1-4(Fucα1-3)GlcNAcβ-]. Thus, the antigenic epitope of SSEA-1 has been considered to be Lex (Note: strictly, “SSEA-1” is not equal to “Lex”. However, in this review we will describe both as SSEA-1 because SSEA-1 and Lex have not been clearly distinguished in the literature discussed in this section. SSEA-1 is also known as CD15 according to the cluster of differentiation (CD) nomenclature.
The expression of SSEA-1 was originally reported as being confined to undifferentiated cells, but not in the differentiated cells (Solter and Knowles 1978, 1979; Knowles et al. 1980). These early reports underline the importance of SSEA-1 as a marker for undifferentiated cells. In fact, it is well known that SSEA-1 is utilized as a specific marker of mouse embryonic stem (ES) cells (Muramatsu and Muramatsu 2004). More recent studies, however, have indicated that SSEA-1 is also expressed in more differentiated tissues and cells. In the CNS, SSEA-1 is abundantly expressed in the developing mouse brain (Fox et al. 1981; Yamamoto et al. 1985; Dasgupta et al. 1996; Ashwell and Mai 1997). Furthermore, SSEA-1 expression was found in human embryonic NSCs (Klassen et al. 2001), in mouse postnatal, adult, and embryonic NSCs/NPCs (Klassen et al. 2001; Capela and Temple 2002; Kim and Morshead 2003; Corti et al. 2005; Yanagisawa, Taga, et al. 2005), and in mouse embryonic retinal progenitor cells (Koso et al. 2006). Because these cells are not fully differentiated, the expression may represent the immature status of the cells as previously suggested. On the other hand, in the developing quail peripheral nervous system (PNS), SSEA-1 is expressed in more mature neural cells, the primary sensory neurons differentiated from neural crest cells (Sieber-Blum 1989).
Taking advantage of the expression pattern and the cell-surface localization of SSEA-1, strategies employing the anti-SSEA-1 antibody have been developed to sort NSCs from brain tissues by flow cytometry. These strategies led to sorting of specific stem-cell populations from the SVZs of adult mouse brain (Capela and Temple 2002; Corti et al. 2005), the germinal zones of embryonic mouse forebrain (Kim and Morshead 2003; Corti et al. 2005), and embryonic mouse retina (Koso et al. 2006). These findings reinforced the concept that SSEA-1 is a specific marker for NSCs/NPCs. The possibility of using SSEA-1 as a marker for selecting human NSCs that express this antigen (Klassen et al. 2001) should also be considered. SSEA-1 expression, however, is not limited to NSCs/NPCs. For this reason, it would be advisable to use anti-SSEA-1 antibody with probes to confirmatory markers or dyes for efficient sorting of NSCs/NPCs, as performed by Kim and Morshead (2003) and Corti et al. (2005).
One of the molecular mechanisms underlying the characteristic SSEA-1 expression pattern in the developing brain has been elucidated. Shimoda et al. (2002) found that SSEA-1 expression was significantly down-regulated in the embryonic brain of a rat small eye strain. In the small eye strain, the activity of fucosyltransferase 9, a key enzyme for SSEA-1 synthesis (Kudo et al. 1998; Nishihara et al. 1999), was also reduced. Because the small eye strain has mutations in the Pax6 transcription factor gene, this study suggests that the expression of SSEA-1 in the developing rat brain is positively regulated by Pax6 via induction of fucosyltransferase 9. Because mouse radial glia and mouse embryonic cortical NPCs express Pax6 (Heins et al. 2002; Abematsu et al. 2006), the expression of SSEA-1 in NSCs/NPCs may also be regulated by Pax6.
As compared with its expression pattern, the functional roles of SSEA-1 in NSCs/NPCs are not well understood. SSEA-1 has been generally considered to be involved in cell adhesion and compaction of the mouse embryo at the morula stage and also in embryonic carcinoma cells (Fenderson et al. 1984; Eggens et al. 1989). In NSCs/NPCs, anti-SSEA-1 antibody does not seem to affect the formation of mouse embryonic neurospheres (floating clonal aggregates formed by NSCs in vitro), but it inhibits cell migration from the neurospheres in experimental conditions (von Holst et al. 2006; Yanagisawa, Taga, et al. 2005). Analysis of mice deficient in fucosyltransferase 9 and SSEA-1 revealed increased anxiety-like behavior of the mice, but no discernible morphological phenotype in brain development (Kudo et al. 2004, 2006). This finding suggests that the function of SSEA-1 may be compensated by other molecules or is not essential for brain development in the mouse embryo.
To determine the functional roles of SSEA-1 in NSCs/NPCs, it is also essential to identify the carrier molecules. SSEA-1 is a common carbohydrate epitope carried by glycoproteins, proteoglycans, and glycolipids. At present, the SSEA-1 epitope has been suggested to be associated with chondroitin sulfate proteoglycans (CSPGs) in mouse embryonic NPCs (Kabos et al. 2004), β1 integrin in mouse embryonic NPCs (Yanagisawa, Taga, et al. 2005), Wnt-1 in mouse embryonic brain (Capela and Temple 2006), and a glycolipid [Galβ1-4(Fucα1-3)GlcNAcβ1-3Galβ1-4Glcβ1-1′Cer] in mouse embryonic NPCs (Yanagisawa, Taga, et al. 2005).
As we discussed, SSEA-1 is expressed in mouse embryonic stem cells (ES cells), but not in human ES cells. Instead of SSEA-1, SSEA-3 [-3GalNAcβ1-3Galα1-4Galβ1-; Kannagi et al. 1983] and SSEA-4 [SSEA-4; NeuAcα2-3Galβ1-3GalNAcβ1-; Kannagi et al. 1983] are expressed in human ES cells and are commonly used as the cell surface markers (for review, see Muramatsu and Muramatsu 2004). Recently, it has been reported that SSEA-4 is expressed in human embryonic NSCs. Piao et al. (2006) found that a small number of cells in the neurospheres, but not in differentiated cells, prepared from human embryonic forebrains (4.5–12 weeks of gestation) and spinal cords (4.5–9.5 weeks of gestation) express SSEA-4 with other ES cell marker proteins, Tra-1-60 and Tra-1-81. Similarly, Barraud et al. (2007) found that SSEA-4-expressing cells are enriched in the NSCs prepared from human embryo forebrains (7–9 weeks of gestation) and the expression is associated with prominin-1 (see Prominin-1). They suggest that a combination of SSEA-4 with prominin-1 can be used for immunological sorting of NSCs from developing human forebrains, as performed for mouse NSCs by Kim and Morshead (2003) and Corti et al. (2005). Further studies to elucidate the molecular properties and functions of SSEA-4 in NSCs remain to be done.
Human natural killer-1 antigen
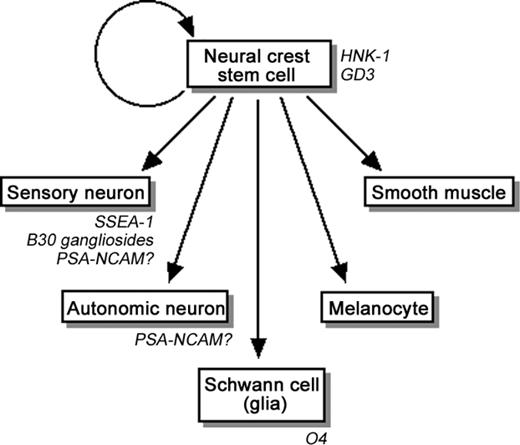
Neural crest cells of the vertebrate embryo are transitory cells that detach from the neural fold of the neural tube and migrate during development to diverse locations (Anderson 1997; Sieber-Blum 2000; Christiansen et al. 2000). Neural crest cells exhibit multipotency (Bronner-Fraser and Fraser 1988) and contain a stem-cell population of neural crest stem cells (Stemple and Anderson 1992; Morrison et al. 1999), which are capable of self-renewal and have the multipotency to differentiate into autonomic neurons by stimulation with bone morphogenetic protein (BMP) 2 (Shah et al. 1996), into Schwann cells by stimulation with glial growth factor (Shah et al. 1994), and into smooth muscles by stimulation with transforming growth factor-β (Shah et al. 1996) (Figure 2).
Multipotency of neural crest stem cells. The glycoconjugate and carbohydrate markers are indicated in italic.
The human natural killer-1 (HNK-1) antigen (CD57) is a carbohydrate antigen whose structure has been established as HSO3-3GlcAβ1-3Galβ1-4GlcNAc- (Chou et al. 1986; Ariga et al. 1987). By studying model compounds, Tokuda et al. (1998) established the minimal carbohydrate epitope structure as HSO3-3GlcAβ1-3Galβ1-. HNK-1 is a well-known cell surface marker that is one of the common antigens in the nervous system (Tucker et al. 1984; Vincent and Thiery 1984; Bronner-Fraser 1986). In the avian and rodent nervous systems, HNK-1 is distributed on the surface of neural crest cells and is required for their proper migration during development (Bronner-Fraser 1987; Holley and Yu 1987; Nagase et al. 2003). Therefore, regulation of neural crest cell migration by HNK-1 may be a general mechanism in all vertebrates. However, Tucker et al. (1988) reported that mouse neural crest cells are negative for HNK-1, indicating that the mouse neural crest cells differ from avian and rat neural crest cells in the expression pattern of the HNK-1 epitope. It remains to be determined whether the requirement of the HNK-1 epitope for mouse neural crest cell migration is compensated for by the presence of other molecules.
Interest in the HNK-1 epitope arises from its association with a number of cell-adhesion molecules (Kruse et al. 1984; Jungalwala 1994). Of the carrier molecules of HNK-1 antigen, glycoproteins [e.g. L1, P0, and neural cell-adhesion molecule (NCAM); Kruse et al. 1984], glycolipids (e.g. SGPG and SGLPG; Chou et al. 1986; Ariga et al. 1987), and proteoglycans (e.g. CSPGs; Domowicz et al. 1995; Pettway et al. 1996) have been characterized. Because of the wide distribution on various glycoconjugates, HNK-1 is expected to play important functional roles in neural development. So far, mice deficient in glucuronyltransferase (GlcAT-P) or HNK-1 sulfotransferase, the key enzymes of HNK-1 antigen synthesis, have been established (Yamamoto et al. 2002; Senn et al. 2002). In these mice, brain development is generally normal; however, adult mice deficient for GlcAT-P or HNK-1 sulfotransferase exhibit reduced long-term potentiation and defective spatial memory formation. These results suggest a functional role of the HNK-1 antigen in synaptic plasticity of the hippocampus, but not in brain development.
More recently, HNK-1 expression in mouse embryonic NPCs was confirmed to be exclusively associated with glycoproteins and/or proteoglycans (Yanagisawa, Taga, et al. 2005). On the other hand, ventrally emigrating neural-tube cells, multipotent cells that also appear from neural tubes but differ from neural crest cells, are reported to lack expression of HNK-1 (Dickinson et al. 2004). At present, the detailed expression pattern, the nature of the carrier glycoproteins/proteoglycans, and the functional roles of HNK-1 in the NPCs are not yet completely elucidated.
Other glycoconjugate markers reported to be present in neural crest and neural crest-derived cells include GD3 ganglioside in mouse neural crest cells (Stainier et al. 1991), SSEA-1 in quail committed sensory neurons (Sieber-Blum 1989), B30 gangliosides (two unidentified gangliosides recognized by the B30 antibody—one migrates slightly above GM1, and the other migrates between GD1a and GD3 on thin-layer chromatography) in mouse sensory neurons (Stainier et al. 1991), polysialic acid-neural cell adhesion molecule (PSA-NCAM) in mouse and rat sensory and/or autonomic neurons (Boisseau et al. 1991; Stemple and Anderson 1992), and O4 antigen (sulfatide) in rat Schwann cells (Stemple and Anderson 1992), and mouse Schwann cells and their precursors (Dong et al. 1999) (Figure 2).
Polysialic acid–neural cell adhesion molecule
Unlike SSEA-1 and HNK-1, the polysialic acid (PSA) carbohydrate structure (Finne et al. 1983) exclusively carried by the neural cell adhesion molecule (NCAM) is expressed in NSCs/NPCs. PSA is a linear homopolymer containing 8–100 α2–8-linked sialic acid residues (SAα2-8SAα2-; Rutishauser and Landmesser 1996), whose synthesis is catalyzed by polysialyltransferases, ST8SiaII (also known as STX) and ST8SiaIV (also known as PST) (Angata and Fukuda 2003). As expected from its characteristic carbohydrate structure, PSA presents interesting features, such as highly negative charges and a high level of hydration, and an excellent ability to bind cations. PSA-NCAM is abundantly expressed during neural development. Although expression is drastically decreased in adult brain, certain regions (e.g., the SVZ, olfactory bulb, and hippocampus) have continuously expressed PSA-NCAM (Angata and Fukuda 2003; Seki and Arai 1993). Taking advantage of the expression pattern, Pennartz et al. (2004) successfully established a method to purify PSA-NCAM-positive NSCs/NPCs from adult mouse brain. It should be noted, however, that cells expressing PSA-NCAM in the postnatal rat brain behave like glial precursor cells in vitro and are mostly committed to a glial fate (Ben-Hur et al. 1998). More detailed studies about the lineage of the PSA-NCAM-positive cells are still needed.
The remarkable chemical structure and expression pattern of PSA-NCAM suggest its importance in brain development. In fact, it has been shown that PSA-NCAM regulates myelination, axon guidance, synapse formation, and functional plasticity of the nervous system. Mice deficient in polysialyltransferase present developmental and behavioral defects, including reduction of long-term potentiation and long-term depression (in ST8SiaIV/PST-deficient mice; Eckhardt et al. 2000) and misguidance of mossy fibers and ectopic synapse formation in the hippocampus (in ST8SiaII/STX-deficient mice; Angata et al. 2004). Surprisingly, no defect in NSCs/NPCs has been reported by these loss-of-function experiments in mice. A gain-of-function experiment by inducing stable over-expression of PSA in mouse NSCs, however, suggests that PSA-NCAM plays an important role during NSC/NPC differentiation and migration; in mouse NSCs/NPCs over-expressing PSA, migration is enhanced and oligodendrocytogenesis is suppressed, although the cells' multipotency and survival are not affected (Franceschini et al. 2004). Furthermore, it has been reported that enzymatic digestion of PSA represses migration and induces premature neuronal differentiation of adult mouse NSCs (Petridis et al. 2004). Thus, it is considered that PSA-NCAM may not be essential for NSCs/NPCs, but that it serves to modify the cell fate possibly by its characteristic chemical structure.
PSA-NCAM is expressed not only in the CNS but also in the PNS. Boisseau et al. (1991) found that expression of high PSA-NCAM was restricted to an early neuronal lineage cells derived from neural crest cells. Recently, it has been reported that BMP enhances migration, neurite fasciculation, and clustering of neuronal cells via promotion of polysialylation of NCAM in the enteric nervous system formed from neural crest cells (Fu et al. 2006; Faure et al. 2007). These studies suggest the importance of regulation of glycoconjugate expression by cytokine signaling in the developing nervous system.
Prominin-1
Prominin-1, also known as CD133 or AC133 (human homolog), is a pentaspan membrane glycoprotein (Shmelkov et al. 2005) originally identified as an antigen expressed on the apical surface of neuroepithelial stem cells and in several other epithelia, including kidney proximal tubules (Weigmann et al. 1997). This antigen, however, has been shown to be expressed not only in embryonic NSCs but also in adult NSCs (Sawamoto et al. 2001), hematopoietic stem cells (Yin et al. 1997) and brain cancer stem cells (Hemmati et al. 2003; Singh et al. 2003), indicating its specificity as a somatic stem-cell marker.
Researchers have taken advantage of the expression pattern on the neuroepithelial cell surface to sort NSCs using flow cytometry with anti-pominin-1 antibody. In 2000, Uchida et al. established a method to isolate NSCs directly from fresh human fetal brains. In their study, cells positive for prominin-1 and negative for CD34 (sialomucin) and CD45 (T200 glycoprotein) were NSCs capable of forming neurospheres and differentiating into both neurons and glial cells. NSCs positive for prominin-1 have also been sorted from mouse postnatal cerebellum (Lee et al. 2005).
Investigators using electromicroscopic analysis have found that prominin-1 is specifically associated with plasma membrane protrusions that have a microvilli-like structure on the apical surface of neuroepithelial cells (Weigmann et al. 1997). Interestingly, during the early stages of neurogenesis, the apical plasma membrane protrusions containing prominin-1 are released to the lumen of the neural tube as a novel class of extracellular membrane particles with diameters of approximately 600 and 50–80 nm (Marzesco et al. 2005). The release of the prominin-1-containing particles is not limited to the developing brain, and the occurrence of the particles is confirmed in various adult human body fluids, including seminal fluid, saliva, urine, and lacrimal fluid. Although the physiological significance of prominin-1 is still unclear, its characteristic localization pattern on the apical plasma membrane and its presence in the novel class of particles suggest that prominin-1 may play important roles in the maintenance of membrane protuberances and cell polarity, and in interaction with the niche. In fact, analysis of a family with autosomal recessive retinal degeneration has revealed that a single nucleotide deletion of a protamin-1 gene causing the frame-shift mutation disrupts transportation of prominin-1 to the cell surface and leads to retinal degeneration due to impaired photoreceptor disk morphogenesis (Maw et al. 2000).
gp130
gp130 (CD130) is a cell surface glycoprotein originally found as a receptor component and signal transducer of interleukin (IL)-6 (Taga et al. 1989; Taga and Kishimoto 1997; Fukuda and Taga 2005). Further studies revealed that this molecule mediates signaling activated by all eight IL-6 family of cytokines, such as IL-11, leukemia inhibitory factor (LIF), ciliary neurotrophic factor (CNTF), oncostatin M, cardiotrophin-1, cardiotrophin-like cytokines/novel neurotrophin-1/B-cell stimulating factor 3, and neuropoietin. Of the signaling pathways activated by the IL-6 family cytokines via gp130, the Janus kinase (JAK)-signal transducer and activator of transcription 3 (STAT3) pathway, the Ras–mitogen-activated protein kinase (MAPK) pathway and the phosphatidylinositol 3 kinase–Akt pathway are known. Although the expression of gp130 is ubiquitous and exhibits no significant pattern in NSCs, the functional aspect is too important to ignore.
Thus far, gp130 has been shown to play critical roles in NSCs/NPCs. First, it is involved in the induction of astrocyte differentiation. It has been reported that the IL-6 family of cytokines induce astrocyte differentiation of rat and mouse embryonic NPCs via activation of a gp130 homo-/hetero-dimer and the JAK–STAT pathway (Bonni et al. 1997, Nakashima, Yanagisawa, et al. 1999; Fukuda and Taga 2005). Among the IL-6 family of cytokines having this activity (Fukuda and Taga 2005; Ohno et al. 2006), cardiotrophin-1 is proposed to be a bona fide inducer for astrocyte differentiation in the developing brain (Barnabe-Heider et al. 2005). The involvement of gp130 in astrocyte differentiation has been confirmed in physiological conditions; gp130-knockout mice exhibit reduction of astrocyte numbers in the developing brain (Nakashima, Wiese et al. 1999). Astrocytic differentiation, however, is not regulated only by the IL-6 family of cytokines, gp130, and the downstream JAK–STAT pathway. For instance, the positive and negative cross-talk of gp130 signaling with BMP signaling (Nakashima, Yanagisawa, et al. 1999), neurogenin-2 (a basic helix-loop-helix [bHLH] transcription factor; Sun et al. 2001), or Notch–hairy-enhancer of split (HES) signaling (Kamakura et al. 2004) has been clarified. In addition, the epigenetic status of astrocytic genes in NPCs is also important for the gp130-mediated astrocyte differentiation (Takizawa et al. 2001).
Secondly, gp130 is involved in maintenance of the self-renewal property of NSCs. Shimazaki et al. (2001) reported that CNTF maintains embryonic and adult NSCs in an undifferentiated state by blocking the differentiation via gp130 signaling. They also found that activation of gp130 leads to an increase in Notch-signaling functioning in NSC maintenance and proliferation (Chojnacki et al. 2003). On the other hand, CNTF is not considered to be a secreted cytokine because of its lack of a secretory signal sequence in the gene and the cytosolic localization of the protein. It should be noted that other IL-6 families of cytokines such as neuropoietin are suggested to share the biological function of CNTF (Derouet et al. 2004). Therefore, the self-renewal property of NSCs may be maintained by multiple IL-6 families of cytokines.
In addition to the above, gp130 signaling has been reported to support NPC survival via activation of the phosphatidylinositol 3 kinase-Akt pathway (Chang et al. 2004). As compared with these diverse biological activities, however, the structure and function of the carbohydrate chain(s) carried by gp130 are totally unknown. The carbohydrate chain of gp130 may be involved in the receptor-ligand interaction, such as that by glycosaminoglycans of proteoglycans.
Chondroitin sulfate proteoglycans
Proteoglycans, the major components of the ECM, are a class of glycosylated proteins having covalently linked glycosaminoglycans, sulfated carbohydrate chains made of repeating disaccharides. Based on the components of disaccharides, proteoglycans are divided into certain subclasses. For instance, proteoglycans having chondroitin sulfate glycosaminoglycans are classified as CSPGs, whereas proteoglycans having heparan sulfate glycosaminoglycans are classified as heparan sulfate proteoglycans (HSPGs). Both subclasses of proteoglycans are known to be expressed in NSCs/NPCs.
As CSPGs expressed in NSCs/NPCs, tenascin C (Garcion et al. 2004; Kabos et al. 2004), aggrecan (Kabos et al. 2004), neurocan (Ida et al. 2006), phosphacan (Kabos et al. 2004; Ida et al. 2006; von Holst et al. 2006), and neuroglycan C (Ida et al. 2006) have been reported. Among these components, the importance of tenascin C and phosphacan has been well studied. Tenascin C is a CSPG robustly expressed in the SVZ and considered to contribute to generation of the stem cell niche. By analyzing tenascin C-knockout mice, Garcion et al. (2004) clearly demonstrated that this CSPG regulates the NSC differentiation via modulation of cytokine signaling in developing mouse brains. Similarly, phosphacan, a CSPG having the glycosaminoglycan epitope recognized by 473HD antibody and representing a neurite outgrowth activity (Faissner et al. 1994), is also localized in the postnatal and adult NSC niche. In considering the characteristic expression pattern of phosphacan, von Holst et al. (2006) isolated cells positive for 473HD from mouse embryonic brain by immunopanning or sorting with magnetic beads, and demonstrated that these 473HD-positive cells exhibit NSC features. Interestingly, modification of 473HD epitope by chondroitinase ABC or 473HD antibody prevented 473HD-positive NSCs from neurosphere formation, suggesting the physiological significance of phosphacan and glycosaminoglycan in NSC self-renewal.
Another important CSPG is nerve/glial antigen 2 (NG2), which was originally identified in rat. The mouse homolog is also known as AG2. NG2 is a CSPG highly expressed in adult and embryonic brains (Stallcup 2002). Cells positive for NG2 have been considered to be committed oligodendrocyte progenitors because they exclusively express oligodendrocyte markers and give rise to oligodendrocyte lineage cells (Dawson et al. 2000; Stallcup 2002). For instance, O-2A progenitor cells, glial precursor cells capable of differentiating into oligodendrocytes and type-2 astrocytes (Raff et al. 1983), are positive for NG2 (Stallcup and Beasley 1987; Levine and Stallcup 1987). It has also been asserted that NG2-glia, an immature glial-cell population capable of giving rise to oligodendrocytes, express this proteoglycan (Nishiyama et al. 2005). More recently, however, it has been reported that NG2-positive cells in postnatal mouse brain exhibit characteristics of NSCs, such as multipotency to differentiate into oligodendrocytes and astrocytes as well as neurons (Belachew et al. 2003; Aguirre and Gallo 2004; Aguirre et al. 2004). In addition, it has been confirmed that neurosphere-forming NSCs prepared from rat telencephalons express NG2 (Ida et al. 2006). In light of these different findings, it is tempting to suggest that there are heterogeneous populations of NG2-positive cells giving rise to different cell types. The bona fide lineages and physiological functions of NG2-positive cells remain to be determined. With respect to its functional roles, NG2 has been shown to have a high affinity for bFGF and platelet-derived growth factor-AA, critical mitogens to NSCs/NPCs and oligodendrocyte progenitor cells (Goretzki et al. 1999). The high affinity for growth factors is reminiscent of the critical roles of HSPGs as a bFGF signal cofactor during brain development (see the Heparan sulfate proteoglycans section). Interestingly, NG2-knockout mice exhibit no defect in hippocampal neurogenesis (Thallmair et al. 2006), suggesting that, unlike other proteoglycans such as HSPGs, NG2 may be nonessential for NSCs/NPCs proliferation.
Recently, it has been reported that mice deficient in an Olig2 bHLH transcription factor exhibit severe defects in NG2-positive cells in developing brains and spinal cords (Ligon et al. 2006). This study indicates that development of NG2-positive cells requires Olig transcription factors, especially Olig2.
Heparan sulfate proteoglycans
NSCs/NPCs express CSPGs as well as HSPGs, the other major component of ECM. Expression of a truncated form of perlecan (Joseph et al. 1996), glypican-4 (K-glypican) (Hagihara et al. 2000; Ford-Perriss et al. 2003), and syndecan (Ford-Perriss et al. 2003) has been demonstrated in the neuroepithelium. Among these, glypican-4 (Hagihara et al. 2000) and syndecan (-1 and -3; Zou et al. 2006) have been shown to be expressed in neurospheres and mouse embryonic NPCs, respectively. Notably, the expression of glypican-4, a glycosylphosphatidylinositol-anchored HSPG, in NPCs disappears immediately after bFGF depletion to induce multilineage differentiation (Hagihara et al. 2000), suggesting the possibility of glypican-4 as a specific and sensitive marker of undifferentiated NSCs. Furthermore, treatment of NPCs with a recombinant glypican-4 partially inhibits bFGF-induced proliferation of NPCs (Hagihara et al. 2000), indicating the involvement of glypican-4 in bFGF signaling in NSCs/NPCs, one of the most important functions of HSPGs in the cells.
At present, HSPGs are known to bind to many factors such as morphogens and mitogens including bFGF, a critical growth factor that sustains multipotency and induces proliferation of NSCs/NPCs. This association suggests the role of HSPGs in mediating signal transduction. For example, bFGF signaling is mediated by receptors, such as FGFR1IIIc and FGFR3IIIc, but an additional cofactor is required for formation of the receptor complex. The cofactor forming a complex with bFGF and FGFR is HSPG (Nurcombe et al. 1993). In NSCs/NPCs, active HSPGs secreted from the cells exhibit high affinity for bFGF and selectivity to different FGF receptors via the heparan sulfate chains (Brickman et al. 1995). The importance of HSPGs for FGF signaling in NPCs/NPCs is further demonstrated in physiological conditions. Brains of conditional knockout mice lacking EXT1, a heparan sulfate (HS)-polymerizing enzyme essential for HS synthesis, exhibit various severe defects, such as thinning of the cortex (Inatani et al. 2003). In NPCs prepared from the knockout mice, bFGF- or fibroblast growth factor 8-induced proliferation is reduced. These reports suggest that HSPGs play a critical role in bFGF-induced proliferation of NSCs/NPCs mediated by highly regulated signaling mechanisms. As a cofactor of HSPG for the bFGF signaling during neurogenesis, a truncated form of perlecan secreted from neuroepithelial cells has been reported (Aviezer et al. 1994; Joseph et al. 1996). In NSCs/NPCs, glypican-4 has also been proposed to act as a potent cofactor for bFGF signaling (Hagihara et al. 2000).
In addition to bFGF, midkine, a heparan-binding growth factor, has also been reported to induce proliferation and survival of mouse embryonic NPCs (Zou et al. 2006). The midkine signaling is mediated by multimolecules including HSPGs. In the NPCs, the importance of HSPGs, such as syndecan-1 and -3, as components of the midkine receptors is proposed (Zou et al. 2006). On the other hand, NPCs prepared from mouse embryo deficient in syndecan-3 are reported to exhibit no defect in proliferation and differentiation (Hienola et al. 2006).
N-glycosylated cystatin C
In addition to HSPGs, another glycoconjugate has been reported as a cofactor of bFGF signaling. Taupin et al. (2000) found that a soluble factor is required for bFGF-induced proliferation of adult rat hippocampus-derived NSCs in conditioned media of the NSC cultures. Purification and characterization of the autocrine/paracrine factor revealed that it is the glycosylated form of cystatin C, a cysteine protease inhibitor belonging to family 2 of the cystatin superfamily. In addition, it also has been reported that cystatin C secreted by mouse embryonic neurospheres enhances differentiation of ES cells to NSCs, probably in cooperation with bFGF (Kato et al. 2006). These reports suggest that cystatin C is an important glycoconjugate in supporting NSC development and maintenance, although the exact physiological role of cystatin C in NSCs remains unclear.
In contrast to these reports, cystatin C has been proposed not be a factor involved in self-renewal of NSCs, but rather it induces astrocyte differentiation of an immature astrocyte cell line (Kumada et al. 2004). At this time, the origin of this functional discrepancy is not yet clear. One possibility could be related to differences in the methodologies used by the two researchers: Kumada et al. (2004) introduced a cDNA library of immature mouse brains to an immature astrocyte cell line to seek astrocyte inducers; on the other hand, Taupin et al. (2000) used conditioned media of an adult rat hippocampus-derived NSC culture to identify an unknown FGF cofactor. Therefore, there is a possibility that cystatin C analyzed in these studies had different glycosylation patterns, which led to the different biological activities of cystatin C. In fact, the N-glycan of cystatin C is found to be important and essential for the mitogenic activity of cystatin C in NSCs (Taupin et al. 2000). Evaluation of the physiological functions of cystatin C remains to be done, but studies regarding cystatin C and HSPG (Nurcombe et al. 1993; Brickman et al. 1995) may reinforce the importance of the carbohydrate chains for the functions of cofactors in bFGF signaling.
In addition to cystatin C, another molecule belonging to the cystatin superfamily, cystatin B, is expressed in mouse embryonic and adult NSCs and differentiated neurons and glial cells from the NSCs (Brannvall et al. 2003).
Galectin-1
Galectin-1 is an animal lectin with Gal-binding specificity (Barondes et al. 1994). Although galectin-1 is not a glycoconjugate, we introduce it as a glycoconjugate-related molecule regulating the fate of NSCs. It has recently been shown that a conditioned medium of OP9 stromal cells contains a factor that enhances neurosphere formation and proliferation of adult mouse NSCs (Sakaguchi et al. 2006). This unknown factor was purified and identified as galectin-1 by tandem mass spectrometry. Further experiments revealed that mouse adult NSCs as well as OP9 cells express galectin-1. Importantly, galectin-1-deficient mice exhibit a decrease of NSCs in the SVZ (Sakaguchi et al. 2006), clearly indicating that galectin-1 plays a role in maintenance of adult NSCs under physiological conditions. The molecular mechanism by which galectin-1 enhances proliferation of the NSCs is unknown. It is tempting, however, to suggest that galectin-1 binds to the carbohydrate chains of signaling molecules and modulates the signal transduction as do HSPGs or and/or cystatin C.
b-series gangliosides
Glycolipids are lipid molecules linked with one or more carbohydrate units. The molecules are classified into glycosphingolipids (GSLs) and glycoglycerolipids based on their core lipid moiety. In their core lipid moiety, GSLs and glycoglycerolipids contain ceramide (Cer) and glycerol, respectively. GSLs containing one or more sialic acid residue(s) are referred to as gangliosides. GSLs are found in virtually all vertebrate cells and body fluids and are particularly abundant in the nervous system. In cells, they are localized primarily, but not exclusively, on the plasma membrane. Although not fully clarified, information regarding the biological functions of GSLs is emerging rapidly. For example, GSLs may serve as mediators in cell–cell recognition and adhesion, as receptors for bioactive molecules, and as modulators of signal transduction (Hakomori 1990; Ledeen and Wu 2002; Hakomori 2003; Yu et al. 2004). In the developing brain, gangliosides are presumed to modulate Cer-induced apoptosis and to maintain cellular survival and differentiation (Bieberich et al. 2001). Furthermore, it is well known that serious neurological deficits develop in animals or humans with aberrant ganglioside metabolism (e.g., various types of glycosphingolipidosis). More recently, Simpson et al. (2004) found that human autosomal recessive infantile-onset symptomatic epilepsy syndrome is associated with a nonsense mutation of GM3-synthase, a key enzyme for synthesis of all complex gangliosides. Furthermore, in the developing brain, the composition of gangliosides undergoes dramatic changes (Yu et al. 1994). Thus, it appears that gangliosides have important biological functions in nervous system development.
Few studies on gangliosides have been devoted to NSCs/NPCs, probably because of the difficulty in isolating relatively pure populations of cells for analysis. The field, however, is now emerging due to the importance of these cell-surface molecules as markers of differentiation and as participants in signal transduction. At present, several gangliosides have been reported to be expressed: GD2 in human embryonic and mouse postnatal NSCs (Klassen et al. 2001), and GD3, GT1b, and GQ1b in mouse embryonic NPCs (Yanagisawa, Nakamura, et al. 2004; Yanagisawa, Taga, et al. 2005). Because all of those gangliosides belong to the so-called b-series (Yu 1994; Yu et al. 2004), it has been proposed that b-series gangliosides can serve as important markers of NSCs/NPCs.
As in the case of SSEA-1, however, the expression of gangliosides is not limited to NSCs/NPCs, which renders them insufficient for cell sorting by anti-ganglioside antibodies alone. Despite this caveat, several investigators have developed flow-cytometric methods for sorting NSCs in brain in an attempt to determine their lineages. Rietze et al. (2001) employed anti-CD24a (heat-stable antigen) antibody and peanut agglutinin, a lectin reactive with a Galβ1-3GalNAc structure, to sort NSCs from periventricular tissue of adult mouse brain. Because this structure is present in the carbohydrate chain of glycoproteins and glycolipids, including GM1 [Galβ1-3GalNAcβ1–4(NeuAcα2–3)Galβ1–4Glcβ1-1′ Cer] and GD1b [Galβ1–3GalNAc1–4(NeuAcα2–8NeuAcα2-3)Galβ1–4Glcβ1–1′ Cer], peanut agglutinin might also be recognizing these gangliosides. Similarly, a recent study by Liour et al. (2005), which used cholera toxin B subunit as a ligand for GM1 and GM1-like gangliosides, revealed that the expression of these gangliosides in mouse embryonic forebrain is restricted to NPCs in the telencephalonic ventricular zone. Therefore, high expression of these gangliosides defines these cells as already differentiated from NSCs. On the other hand, Maric et al. (2003) established a method to sort NSCs from rat embryonic brains by negative selection using flow cytometry. They employed a combination of probes recognizing GSLs. Neuronal-glial precursor cells are labeled with Jones monoclonal antibody (recognizing 9-O-acetyl GD3) (Blum and Barnstable 1987) and A2B5 monoclonal antibody (recognizing c-series gangliosides) (Eisenbarth et al. 1979; Saito et al. 2001); neuronal precursor cells and postmitotic neurons are labeled with a cholera toxin B subunit (reactive with GM1 and the related gangliosides) (Holmgren et al. 1973; King and van Heyningen 1973) and tetanus toxin C fragment (reactive with GD1b ganglioside) (van Heyningen 1980). Maric et al. (2003) have shown that NSCs are cells negative for these GSL markers. Using this method, Maric et al. succeeded in sorting NSCs by negative selection and lineage-restricted precursors by positive selection.
Concerning the functional roles of gangliosides in NSCs/NPCs, more information is known on the involvement in proliferation of GD3 (NeuAcα2–8NeuAcα2–3Galβ1–4Glcβ1-1′ Cer; CD60a), a disialoganglioside belonging to the b-series of gangliosides. Although GD3 is found in Muller cells and neuronal cells in rat and chick retina (Seyfried et al. 1982; Daniotti et al. 1992; Panzetta and Allende 2000), GD3 is known to be abundantly expressed in immature neuroectodermal cells of developing brain (Goldman et al. 1984; Yu et al. 2004) and in rapidly dividing melanoma cells (Carubia et al. 1984) and brain tumor cells (Yates et al. 1979; Traylor and Hogan 1980; Seyfried and Yu 1985). For this reason, GD3 is considered to be involved in cell proliferation. In fact, studies have demonstrated that inhibition or induction of GD3 expression significantly affects the cell proliferation rate of melanoma cells and PC12 cells (Birkle et al. 1999, 2000; Fukumoto et al. 2000). A similar phenomenon has been shown in C17.2 cells, an immortalized NSC line derived from neonatal mouse cerebellar cortex (Ryder et al. 1990). C17.2 cells originally express only a-series gangliosides (Suetake et al. 2003); in cells overexpressing GD3-synthase, however, there is an ectopic expression of b- and c-series gangliosides with concomitant repression of cell proliferation without loss of viability (Yanagisawa, Liour, et al. 2004). Although this result seems contradictory to the above reports, it is thought that overexpression of GD3 restricted cell proliferation of the NCS line. Similarly, in primary mouse NPCs treated with d-threo-1-phenyl-2-decanoylamino-3-morpholino-1-propanol, a potent inhibitor for biosynthesis of all glucosylceramide (GlcCer)-based GSL (Inokuchi and Radin 1987), GD3 expression disappears and bFGF-induced proliferation is retarded (Yanagisawa, Nakamura, et al. 2005). Interestingly, in both cases activation of the Ras–MAPK pathway, a signaling pathway important for NSC/NPC proliferation, is also repressed. These results suggest that the expression level of GD3 ganglioside may be involved in NSC/NPC proliferation by regulating Ras–MAPK signaling.
With respect to the molecular mechanism underlying repression of the Ras–MAPK pathway, involvement is expected of glycolipid-enriched plasma membrane microdomains. These microdomains, also frequently referred to as caveolae (Anderson 1998), lipid rafts (Simons and Ikonen 1997; Simons and Toomre 2000), or glycolipid-enriched microdomains (Hakomori et al. 1998), are rich in certain lipids, such as cholesterol, sphingomyelin, and GSLs. In the microdomains, signaling and cell adhesion molecules are localized, implicating that these domains may form platforms for signal transduction and cell adhesion. Support for this notion came from a recent study showing that in the microdomains of mouse embryonic NPCs, a cell adhesion molecule (β1 integrin) and several signaling molecules (gp130, JAK1, Grb2/SOS, and Ras) are localized (Yanagisawa, Nakamura, et al. 2004; Yanagisawa et al. 2005). Treatment of the NPCs with methyl-β-cyclodextrin (MBCD), a drug that binds to cholesterol and disrupts the functions of microdomains (Scheiffele et al. 1997), represses the integrin-mediated cell adhesion and cytokine-activated Ras–MAPK pathway of NPCs. Therefore, it is assumed that GD3 may be involved in NPC proliferation by modulating the Ras–MAPK pathway in the cell surface microdomains. How microdomain disruption and GSL disturbance repress the Ras–MAPK pathway is still unknown. Presumably, β1 integrin, an important marker molecule and ECM receptor of NSCs (Hall et al. 2006), whose function is disrupted by MBCD treatment (Yanagisawa, Nakamura, et al. 2004) may be involved, because β1 integrin promotes cytokine-induced Ras–MAPK pathway activation in NSCs (Campos et al. 2004; Leone et al. 2005).
Surprisingly, it has been recently found that NPCs prepared from telencephalons of GD3-synthase-deficient mouse embryos represent no phenotypes, even for proliferation and signal transduction (Yu and Yanagisawa 2007). To elucidate the functional roles of GSLs in NSCs/NPCs, further analysis is needed of other genetically engineered animals, such as knockout and transgenic mice. At present, with the advent of molecular and cell biological techniques, many genetically engineered mice lacking the expression of a specific population of GSL(s) have been established (Furukawa et al. 2001; Allende and Proia 2002; Kolter et al. 2002). Analysis of NSCs/NPCs prepared from these mice, especially mice lacking most complex GSLs, such as GlcCer-synthase-conditional knockout mice (Jennemann et al. 2005) or 3-phosphoglycerate dehydrogenase-knockout mice (Yoshida et al. 2004), should offer a new avenue of fruitful research.
A2B5 antigens and O1/O4 antigens
As we discussed in the b-series gangliosides section, GSLs including gangliosides are considered to be useful cell-surface markers for monitoring NSCs/NPCs and their progeny. However, they also have been utilized as specific markers to define oligodendrocyte lineage cells during development.
The first GSL antigen expressed in an oligodendrocyte lineage is the A2B5 antigen. A2B5 is a monoclonal antibody originally developed by Eisenbarth et al. (1979) using embryonic retina cells as the immunogen. The antigens recognized by the A2B5 monoclonal antibody were established as the c-series gangliosides including GQ1c, GT1c, and GT3 (Kasai and Yu 1983; Saito et al. 2001), although they had once been proposed to be b-series gangliosides (GD2, GD3, and GQ1b; Kundu et al. 1983) or sulfatide (Majocha et al. 1989). These c-series gangliosides are abundant in fish brains (Ando and Yu 1979; Yu and Ando 1980; Freischutz et al. 1995), and embryonic, but not adult mammalian brains (Rosner et al. 1988; Freischutz et al. 1994). During development, A2B5 antigens are expressed in common rat glial precursor cells, known as O-2A progenitor cells, which differentiate into type-2 astrocytes and oligodendrocytes (Raff et al. 1983). These antigens are expressed not only in rat O-2A progenitor cells but also in human fetal glial precursor cells (Dietrich et al. 2002). Since the A2B5 antigens are localized on the cell surface, the antibody has also been widely used to isolate the precursor cells by immunopanning (Barres et al. 1992).
As oligodendrocyte development proceeds, other GSLs appear on the cell surface. These GSLs include the O4 antigen (sulfatide; HSO3-3Galβ1-1′ Cer) and the O1 antigen (galactosylCeramide; GalCer; Galβ1-1′ Cer), which are also useful markers to define immature and mature oligodendrocytes, respectively (Zhang 2001). In addition, the O1 and O4 antigens play roles as modulators of oligodendrocyte development and function. Because O1 and O4 antigens are major components of the myelin sheath, their involvement in nerve conduction has been expected. In fact, a series of studies have clearly shown that knockout mice lacking GalCer-synthase (Bosio et al. 1996; Coetzee et al. 1996) or sulfatide-synthase (Honke et al. 2002) present severe tremor, mild ataxia, and conduction deficits, with reduction of nerve conduction velocity. Furthermore, it has been found that the number of oligodendrocytes is increased in sulfatide-synthase-knockout mice (Hirahara et al. 2004), indicating the importance of the O4 antigen for terminal differentiation of oligodendrocytes. As a factor inducing O1 antigen expression via regulation of GalCer-synthase, GalCer expression factor-1, a rat homolog of hepatocyte growth factor-regulated tyrosine kinase substrate, has been cloned (Ogura et al. 1998). Although it is expected that this molecule may regulate the expression of O1 and O4 antigens during development, analysis of the GalCer expression factor-1 in NSCs/NPCs and glial precursor cells remains to be done.
Phosphatidylglucoside and GM3/GD3 gangliosides
Previously, a novel phosphoglycerolipid with an interesting structure, phosphatidylglucoside (PtdGlc), was found in human cord-blood cells (Nagatsuka et al. 2001). PtdGlc is also expressed in a promyelocytic leukemia cell line, HL60 cells, and is localized in the lipid rafts, suggesting that this lipid is critical for organization of the microdomains involved in cellular differentiation of HL60 cells (Nagatsuka et al. 2003). Recently, a DIM21 monoclonal antibody recognizing PtdGlc was established (Yamazaki et al. 2006). Immunocytochemical analysis using the DIM21 monoclonal antibody reveals that PtdGlc is exclusively expressed in astrocytes and radial glia in rat embryonic brain (Nagatsuka et al. 2006). It would be critical to examine the expression of PtdGlc in GFAP-positive astrocytes in SVZ that have been known as adult NSCs.
In addition to PtdGlc, GM3 (an a-series ganglioside) and GD3 (a b-series ganglioside) are expressed in astrocytes prepared from neonatal rat brains (Murakami et al. 1999). Interestingly, these gangliosides exhibit quite different expression patterns between type-1 and type-2 astrocytes: GM3 is restricted to type-1 astrocytes, and GM3 and GD3 are both in type-2 astrocytes. Furthermore, the amount of neutral glycolipids expressed in type-1 astrocytes is higher than that in type-2 astrocytes. Taking advantage of the characteristic expression patterns of these glycolipids, PtdGlc, GM3, GD3, and neutral glycolipids, it may be possible to delineate clearly the difference between the SVZ astrocytes (adult NSCs) and “classical” astrocytes.
O-Fucose glycan of Notch
Notch receptors (Notch in Drosophila; Notch 1–4 in mammals) are transmembrane proteins that regulate a wide range of developmental processes (Artavanis-Tsakonas et al. 1999). In NSCs/NPCs, Notch signaling has been known to inhibit neuronal and oligodendroglial differentiation, to maintain the NSC/NPC pool, and to promote astroglial differentiation (Gaiano and Fichell 2002). Notch signaling is activated by an interaction with ligand molecules, Delta in Drosophila and Delta 1, 3, and 4 in mammals, or Serrate in Drosophila and Jagged 1 and 2 in mammals. In this signaling process, Fringe (lunatic fringe in Drosophila; manic fringe and radical fringe in mammals) plays roles as a promoter of Delta–Notch signaling and as an inhibitor of Serrate–Notch signaling in Drosophila wing (Panin et al. 1997).
It is known that the Notch receptor carries a carbohydrate chain, O-fucose (Fuc) glycan, on the serine or threonine residues within the EGF domains (Haines and Irvine 2003). These O-fucose glycans have important roles in regulating Notch signaling (Haines and Irvine 2003). The synthesis of O-Fuc glycan is initiated by O-linked fucosylation of serine or threonine residues catalyzed by O-fucosyltransferase. RNA interference of Drosophila O-fucosyltransferase 1 leads to defects in Notch signaling, indicating the importance of O-Fuc or O-Fuc glycan in this process (Okajima and Irvine 2002). Mice deficient in O-fucosyltransferase 1 (a mouse homolog of Drosophila fucosyltransferase) also present phenotypes as other mutants for Notch-signaling molecules (Shi and Stanley 2003), suggesting that O-Fuc modification is conserved in various animal species. Interestingly, in addition to its role in glycosylation, O-fucosyltransferase 1 has been reported to have a distinct function as a molecular chaperone of Notch molecules (Okajima et al. 2005).
Some O-Fuc residues are further modified by a series of glycosyltransferases, including β1-3 N-acetylglucosaminyltransferase, β1-4 galactosyltransferase, and α2-3 sialyltransferase. By activities of these enzymes, O-Fuc glycan (SAα2-3Galβ1-4GlcNAcβ1-3Fuc-Ser/Thr) is synthesized by sequential addition of sugar residues (Moloney, Shair et al. 2000). Interestingly, Fringe, a promoter of Delta and an inhibitor of Serrate, has N-acetylglucosaminyltransferase activity and is required for modulation of Notch signaling (Bruckner et al. 2000; Moloney, Panin et al. 2000). Because the elongated O-Fuc glycans leads to a higher affinity Notch for Delta than for Serrate (Okajima et al. 2003), the distinct promoter activities of Fringe for Delta and inhibitor activities for Serrate are presumed to be due to modulation of the Notch–ligand interaction via the elongated O-Fuc glycans. Although there is a possibility that Fringe is not required for CNS development, these studies clearly indicate the importance of carbohydrate chains in Notch signaling. It will be important to elucidate whether O-Fuc glycans have any role in NSCs/NPCs.
Closing remarks
Progress in NSC biology has been tremendous during the last one and half decade. Basic biological studies have elucidated the existence and localization of NSCs in the embryonic and adult brain, and also have supplied fascinating findings about characteristics of NSCs. Meanwhile, cell therapy for regeneration and/or repair by transplanting NSCs into injured regions of the brain for neurodegenerative diseases or into the spinal cord for spinal cord injuries has gained momentum in the clinical arena. The potential of NSC therapy and neural cell replacement in injured regions is enormous. However, a basic question remains, which needs to be solved as soon as possible: what is an NSC?
Recently, researchers, especially neuroscientists, have tended to use the term, “NSCs,” to refer, loosely, to more mature cell lineages. In our opinion, some of those individuals have confused the multipotency of NSCs with the ability of NSCs to “transdifferentiate” from one lineage to another. Because of these factors, we cannot completely ignore the possibility that some cell populations previously considered NSCs in some reports do not have NSC features. In fact, some studies supporting this possibility have been reported. For instance, Bull and Bartlett (2005) insist that adult mouse hippocampal progenitor cells are capable of proliferation and differentiation but are unable to self-renew. Regarding embryonic cells, Mukouyama et al. (2006) showed that most neuroepithelial motoneuron progenitors positive for Olig2 do not have the properties of multipotency and self-renewal in vivo. Therefore, we advocate that it is essential to define NSCs in an unambiguous manner. For this reason, a method to define NSCs in vivo and in vitro is definitely required. For instance, we can refer to the studies of hematologists and immunologists, who in defining hematopoietic stem cells frequently take advantage of a method to inject the candidate cells into irradiated animals and then evaluate their ability to reorganize the whole blood system. Unfortunately, we cannot use this excellent strategy for NSCs because it is not possible to damage the whole nervous system and reconstruct it in living animals. Instead, we are compelled to rely on indirect techniques, such as evaluation of self-renewal by a neurosphere assay and of multipotency by inducing differentiation followed by a cell staining method using specific markers. In the latter case, a number of molecules are already utilized as markers of NSCs. However, as compared with the well-characterized “CD” markers of hematopoietic cell lineages, a system of biomarkers defining NSCs has not yet been well established, resulting in occasional confusion of interpretation. For example, some of the biomarkers exhibit spatiotemporally broad expression patterns. Nestin (Lendahl et al. 1990), a hallmark for characterizing NSCs, is also expressed in the differentiated progeny of NSCs. Platelet-derived growth factor receptor α, reported as a cell-surface marker of adult NSCs (Jackson et al. 2006), is also expressed during development in oligodendrocyte precursors (Zhang 2001). Furthermore, nestin and Sox2 (Graham et al. 2003), an intracellular protein and a transcription factor, respectively, are not suitable as surface markers for labeling living NSCs for defining cell populations. For these reasons, some of the so-called “markers” are not sufficiently specific for use in defining NSCs with certainty.
On the other hand, glycoconjugates are usually localized on the cell surface, and their expression pattern often changes drastically during development. For instance, qualitative and quantitative changes in ganglioside expression in the nervous system correlate with cellular events: emergence of GD3 during neural tube formation; peak expression and synthesis of GM3, GD3, and O-acetyl GD3, accretion of lacto-series gangliosides 3′-LM1, 3′-isoLM1, and 3′,8′-LD1, and emergence of c-series gangliosides (A2B5 antigens) during neuroblast and glioblast proliferation; diminished expression of GD3 and c-series gangliosides, followed by increased synthesis of b-series gangliosides during neurogenesis and neuritogenesis; increased synthesis of a-series gangliosides, particularly GM1 and GD1a, during axonal and dendritic arborization; and expression and accretion of GalCer (O1 antigen) and sulfatide (O4 antigen) during myelination (Yu 1994). Taking advantage of the characteristic and diverse expression patterns of glycoconjugates, we may be able to define NSCs and their progeny clearly. In fact, as mentioned in this review, many kinds of probes, such as antibodies, lectins, and bacterial toxins that recognize carbohydrate antigens or glycoconjugates have already been used for cell staining to identify NSCs/NPCs or glial precursor cells.
Care, however, must be exercised when identification of NSCs/NPCs is based solely on cell-staining techniques, including immuno-/histo-chemistry and flow cytometry. For instance, cholera toxin B subunit, a well-known ligand to detect GM1 ganglioside, has been recently reported to over-react with an extremely low amount of GM1 in mouse embryonic NPCs (Yanagisawa et al. 2006a). Furthermore, the cholera toxin B subunit has a wide range of cross-reactivity; in addition to GM1, a wide variety of glycolipids, such as fucosyl-GM1 (Yanagisawa et al. 2006b), and non-glycolipids including polyphenols and lipid A (Usuki et al. 2007) interact with the cholera toxin B subunit. Therefore, in our opinion, all cell staining-dependent studies should be confirmed by biochemical analysis to avoid overinterpretation and mischaracterization of antigens. Solving these problems in cell staining techniques and delineating the spatiotemporal expression pattern and level of glycoconjugates in neural cells, including NSCs in adult and embryonic brains, may bring about more effective strategies for defining NSCs in an unambiguous manner, distinguishing them from their differentiated progeny and characterizing them more systematically. Solving these problems may also lead to a breakthrough in establishing better methods to isolate bona fide NSCs efficiently for biological and clinical studies.
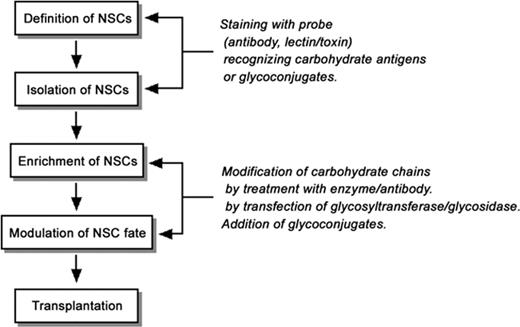
In addition, glycoconjugates are considered to be useful not only for defining and isolating NSCs by cell staining with anti-carbohydrate probes but also for other purposes such as cell therapy by transplantation (Figure 3). Modifying glycoconjugate expression or functions may enable enrichment of NSCs and modulation of NSCs to differentiate into either neuronal or oligodendroglial lineages, which should prove to be beneficial for functional synapse formation and remyelination after transplantation (Figure 3). Since cell therapy by transplantation of NSCs requires a number of NSCs, enrichment of the cells after isolation is considered essential. As we discussed in this review, a wide variety of glycoconjugates including proteoglycans are known to regulate NSC fate, including proliferation. Therefore, it may be possible to promote proliferation of NSCs by modifying these glycoconjugates. Furthermore, glycoconjugates may enable induction of differentiation of NSCs into specific-cell lineages beneficial for neuroregeneration after transplantation (Figure 3). Transplanted NSCs tend to differentiate, not into neurons or oligodendrocytes, but predominantly into astrocytes that inhibit functional recovery of the injured regions. Especially in the spinal cord, glial scars formed after injury to astrocytes severely inhibit regeneration and remyelination of axons. In this process, glycoconjugate modification or treatment may be able to change the fate of NSCs and induce their differentiation into neurons or oligodendrocytes. Similarly, usage of glycoconjugates may enable preferential induction of the migration of transplanted cells to appropriate regions for remyelination, functional synapse formation, neurotrophic factor delivery, and axon guidance after transplantation (Figure 3). In fact, it has been reported that overexpression of PSA by infection of ST8SiaIV in astrocytes promotes recruitment of NSCs/NPCs and regeneration of axons in experimentally injured regions and may lead to neural tissue repair (El Maarouf et al. 2006).
A model strategy of using glycoconjugates and carbohydrate antigens for NSC transplantation therapy. It is thought that glycoconjugates and carbohydrate antigens are useful markers to define and isolate NSCs by staining with specific cell surface probes. In addition, it may be possible to modulate the fate of NSCs by modifying the carbohydrate chains involved in signal transduction or cell adhesion.
Furthermore, NSCs/NPCs over-expressing certain glycosyltransferases by genetic engineering are considered to contribute to the treatment of neural disorders such as lysosomal storage diseases (Figure 3). Lysosomal storage diseases (or lysosomal diseases) are defined as a group of inherited disorders caused by the accumulation of undegraded substrates such as glycoconjugates in the lysosomes. As the typical lysosomal storage diseases associated with neuropathological symptoms, gangliosidoses (including Tay–Sachs disease and Sandhoff disease), Fabry disease, metachromatic leukodystrophy, Krabbe disease, and mucopolysaccharidoses are known. Because the accumulation of glycoconjugates causing onset of the diseases results from the gene defects of lysosomal glycosidases and cofactors, enzyme replacement therapy has been considered to be one of the possible treatments. So far, usage of NSCs/NPCs genetically engineered to over-express glycosidases has been attempted for the enzyme replacement therapy of the lysosomal storage diseases. For instance, NSCs/NPCs or the NSC lines expressing β-hexosaminidase α-subunit (for Tay–Sachs disease; Lacorazza et al. 1996), β-galactosylceramidase (for Krabbe disease; Torchiana et al. 1998), or β-glucuronidase (for mucopolysaccharidosis type VII; Buchet et al. 2002) have been prepared and evaluated for the efficiency of transplantation to the animals. More importantly, transplantation of genetically engineered mouse NSC line or human embryonic NSCs over-expressing β-glucuronidase has been reported to reduce lysosomal storage in the brains of mucopolysaccharidosis type VII model mice (Snyder et al. 1995; Meng et al. 2003; Eto et al. 2004). Similarly, mouse embryonic NSCs overexpressing arylsulfatase A has also been shown partially to clear the accumulated glycoconjugate, sulfatide, in a mouse model of metachromatic leukodystrophy (Kawabata et al. 2006). Moreover, it has been reported that transplantation of mouse potnatal NSCs or mouse NSC lines overexpressing β-galactosylceramidase leads to delayed onset of the symptoms, reduced psychosine levels, enhanced myelination, and longer lifespan in mice with Krabbe disease (Taylor et al. 2006; Pellegatta et al. 2006). Although transplantation of mouse embryonic NSCs even without genetic engineering has also been shown to clear the lysosomal storage and improve the behavioral patterns of mucopolysaccharidosis type VII model mice (Fukuhara et al. 2006), the strategy to modify the endogenous glycoconjugate expression by NSC/NPC transplantation may be possible for treatment or prevention of other glycoconjugate-related neural disorders, such as Alzheimer's disease whose onset is regulated by GM1 ganglioside (Hayashi et al. 2004).
As we discussed, studies about glycoconjugates expressed in NSCs contribute to defining NSCs and using these cells for clinical purposes, but these studies may also elucidate the functional roles of NSCs. The importance of the niche for development and functionality of NSCs has recently been well recognized. These studies have reinforced the notion that NSCs are regulated by more highly diverse mechanisms, such as signaling from soluble factors including mitogens and morphogens, epigenetic modulation, and cell–cell interaction via adhesion molecules and ECM. In fact, as we have discussed in this review, HSPG and glycosylated cystatin C, and perhaps GSLs, modulate the signaling pathway triggered by bFGF, an important mitogen of NSCs/NPCs. At present, our understanding of the functional roles of glycoconjugates in NSCs is still fragmentary and incomplete. Future work should be most rewarding if we seek a better understanding of the functions of glycoconjugates expressed in NSCs and unravel their biological functions.
Acknowledgments
This work was supported in part by grants from National Institutes of Health (NS11853, AG 027199, and NS26994) and a grant from the Children's Medical Research Foundation. We thank Ms Diana Westbrook for her expert editorial assistance. We also thank Ms Sathaporn Ngamukote for her technical help.
Conflict of interest statement
None declared.
Abbreviations
- bFGF
basic fibroblast growth factor
- bHLH
basic helix–loop–helix
- BMP
bone morphogenetic protein
- CD
cluster of differentiation
- Cer
ceramide
- CNS
central nervous system
- CNTF
ciliary neurotrophic factor
- CSPG
chondroitin sulfate proteoglycan
- ECM
extracellular matrix
- EGF
epidermal growth factor
- ES cell
embryonic stem cell
- Fuc
fucose
- Gal
galactose
- GalCer
galactosylceramide
- GalNAc
N-acetylgalactosamine
- GFAP
glial fibrillary acidic protein
- GlcA
glucuronic acid
- GlcCer
glucosylceramide
- GlcNAc
N-acetylglucosamine
- GSL
glycosphingolipid
- HES
hairy-enhancer of split
- HNK-1
human natural killer-1
- HSPG
heparan sulfate proteoglycan
- IL
interleukin
- JAK
Janus kinase
- MAPK
mitogen-activated protein kinase
- MBCD
methyl-ß-cyclodextrin
- NCAM
neural cell adhesion molecule
- NeuAc
N-acetylneuraminic acid
- NG2
nerve/glial antigen 2
- NPC
neural precursor cell
- NSC
neural stem cell
- PNS
peripheral nervous system
- PSA
polysialic acid
- PtdGlc
phosphatidylglucoside
- SA
sialic acid
- SSEA-1
stage-specific embryonic antigen-1
- SSEA-4
stage-specific embryonic antigen-4
- STAT3
signal transducer and activator of transcription 3
- SVZ
subventricular zone. Ganglioside nomenclature is based on that of Svennerholm (1963)