Abstract
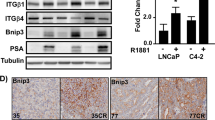
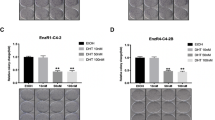
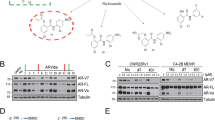
Resistance to antiandrogen therapy in patients with metastatic prostate cancer poses a major challenge, which, if overcome, may lead to significant advances in the treatment of these patients. Hormone resistance of prostate cancer develops, in part, from upregulation of antiapoptotic genes after androgen deprivation. Given the accumulating evidence that Survivin, a new member of the inhibitor of apoptosis (IAP) family, is associated with both cancer progression and drug resistance, we hypothesized that Survivin plays a potentially important role in hormone therapy resistance, and that targeting of Survivin may enhance sensitivity to antiandrogen therapy in prostate cancer. Patterns of Survivin expression were assessed in three prostate cancer cell lines LNCaP, PC-3, and DU-145 using quantitative Western analysis. All three cell lines were found to strongly express Survivin. In LNCaP cells with intact androgen receptors (ARs), it was observed that androgen stimulation with 5α-dihydrotestosterone (DHT) increased Survivin expression. Conversely, treatment with Flutamide decreased Survivin expression in LNCaP cells. We next studied the functional effect of Survivin on sensitivity to Flutamide. LNCaP cells were infected with replication-deficient adenoviruses encoding either wild-type Survivin pAd-S(WT) or a phosphorylation-defective Survivin Thr34 → Ala dominant-negative mutant pAd-S(T34A), and then treated with Flutamide. Cell viability and apoptosis were assessed in vitro and in vivo. It was determined that Survivin can mediate resistance to such antiandrogen therapies based on our assays. Direct androgen stimulation resulted in pan-cell cycle expression of Survivin, which was found to be mediated by AKT, as it was determined that exogenous insulin-like growth factor-1 (IGF-1), a known activator of AKT signaling, could increase Survivin expression and result in pan-cell cycle expression even in AR-negative prostate cancer cell lines PC-3 and DU-145. Given this alternative mechanism of Survivin expression and our findings that Survivin can mediate resistance to Flutamide treatment, we further investigated whether IGF-1-mediated activation of Survivin via AKT could mediate resistance to antiandrogen therapy. Both in vitro and in vivo, this was found to be the case, supporting a novel mechanism of resistance to antiandrogen therapy. Our study indicates that upregulation of Survivin via IGF-1 signaling confers resistance to Flutamide in prostate cancer cells. Targeted inhibition of Survivin appears to enhance the therapeutic effects of Flutamide in vitro and in vivo, revealing a novel strategy to enhance sensitivity to androgen ablation therapy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Altieri DC . (2001). Trends Mol. Med., 7, 542–547.
Altieri DC . (2003a). Oncogene, 22, 8581–8589.
Altieri DC . (2003b). Nat. Rev. Cancer, 3, 46–54.
Ambrosini G, Adida C and Altieri DC . (1997). Nat. Med., 3, 917–921.
Baserga R, Peruzzi F and Reiss K . (2003). Int. J. Cancer, 107, 873–877.
Burfeind P, Chernicky CL, Rininsland F and Ilan J . (1996). Proc. Natl. Acad. Sci. USA, 93, 7263–7268.
Craft N, Shostak Y, Carey M and Sawyers CL . (1999). Nat. Med., 5, 280–285.
Denmeade SR and Isaacs JT . (2002). Nat. Rev. Cancer, 2, 389–396.
Djavan B, Waldert M, Seitz C and Marberger M . (2001). World J. Urol., 19, 225–233.
Dudek H, Datta SR, Franke TF, Birnbaum MJ, Yao R, Cooper GM, Segal RA, Kaplan DR and Greenberg ME . (1997). Science, 275, 661–665.
Feldman BJ and Feldman D . (2001). Nat. Rev. Cancer, 1, 34–45.
Fukuda S, Foster RG, Porter SB and Pelus LM . (2002). Blood, 100, 2463–2471.
Goldspiel BR and Kohler DR . (1990). DICP, 24, 616–623.
Horoszewicz JS, Leong SS, Kawinski E, Karr JP, Rosenthal H, Chu TM, Mirand EA and Murphy GP . (1983). Cancer Res., 43, 1809–1818.
Isaacs JT . (1999). Urol. Clin. North Am., 26, 263–273.
Jemal A, Tiwari RC, Murray T, Ghafoor A, Samuels A, Ward E, Feuer EJ and Thun MJ . (2004). CA Cancer J. Clin., 54, 8–29.
Kassen AE, Sensibar JA, Sintich SM, Pruden SJ, Kozlowski JM and Lee C . (2000). Prostate, 44, 124–132.
Kim IY, Zelner DJ, Sensibar JA, Ahn HJ, Park L, Kim JH and Lee C . (1996). Exp. Cell Res., 222, 103–110.
Kishi H, Igawa M, Kikuno N, Yoshino T, Urakami S and Shiina H . (2004). J. Urol., 171, 1855–1860.
Koivisto P, Kononen J, Palmberg C, Tammela T, Hyytinen E, Isola J, Trapman J, Cleutjens K, Noordzij A, Visakorpi T and Kallioniemi OP . (1997). Cancer Res., 57, 314–319.
Kulik G, Klippel A and Weber MJ . (1997). Mol. Cell. Biol., 17, 1595–1606.
Kyprianou N, English HF and Isaacs JT . (1990). Cancer Res., 50, 3748–3753.
Lee C, Sutkowski DM, Sensibar JA, Zelner D, Kim I, Amsel I, Shaw N, Prins GS and Kozlowski JM . (1995). Endocrinology, 136, 796–803.
Li F . (2003). J. Cell. Physiol., 197, 8–29.
Li F and Altieri DC . (1999). Cancer Res., 59, 3143–3151.
Limonta P, Dondi D, Marelli MM, Moretti RM, Negri-Cesi P and Motta M . (1995). J. Steroid Biochem. Mol. Biol., 53, 401–405.
Ling X, Bernacki RJ, Brattain MG and Li F . (2004). J. Biol. Chem., 279, 15196–15203.
Marcelli M, Ittmann M, Mariani S, Sutherland R, Nigam R, Murthy L, Zhao Y, DiConcini D, Puxeddu E, Esen A, Eastham J, Weigel NL and Lamb DJ . (2000). Cancer Res., 60, 944–949.
McEleny KR, Watson RW, Coffey RN, O'Neill AJ and Fitzpatrick JM . (2002). Prostate, 51, 133–140.
Mesri M, Wall N, Li J, Kim R and Altieri DC . (2001). J. Clin. Invest., 108, 981–990.
Nickerson T, Chang F, Lorimer D, Smeekens SP, Sawyers CL and Pollak M . (2001). Cancer Res., 61, 6276–6280.
O'Connor DS, Wall NR, Porter AC and Altieri DC . (2002). Cancer Cell, 2, 43–54.
Oh WK and Kantoff PW . (1998). J. Urol., 160, 1220–1229.
Okamoto M, Lee C and Oyasu R . (1997). Endocrinology, 138, 5071–5074.
Papapetropoulos A, Fulton D, Mahboubi K, Kalb RG, O'Connor DS, Li F, Altieri DC and Sessa WC . (2000). J. Biol. Chem., 275, 9102–9105.
Parrizas M, Gazit A, Levitzki A, Wertheimer E and LeRoith D . (1997). Endocrinology, 138, 1427–1433.
Peruzzi F, Prisco M, Dews M, Salomoni P, Grassilli E, Romano G, Calabretta B and Baserga R . (1999). Mol. Cell. Biol., 19, 7203–7215.
Remacle-Bonnet MM, Garrouste FL, Heller S, Andre F, Marvaldi JL and Pommier GJ . (2000). Cancer Res., 60, 2007–2017.
Ruijter E, van de Kaa C, Miller G, Ruiter D, Debruyne F and Schalken J . (1999). Endocr. Rev., 20, 22–45.
Shariat SF, Lotan Y, Saboorian H, Khoddami SM, Roehrborn CG, Slawin KM and Ashfaq R . (2004). Cancer, 100, 751–757.
Shen R, Sumitomo M, Dai J, Harris A, Kaminetzky D, Gao M, Burnstein KL and Nanus DM . (2000). Endocrinology, 141, 1699–1704.
Taplin ME, Bubley GJ, Ko YJ, Small EJ, Upton M, Rajeshkumar B and Balk SP . (1999). Cancer Res., 59, 2511–2515.
Tran J, Master Z, Yu JL, Rak J, Dumont DJ and Kerbel RS . (2002). Proc. Natl. Acad. Sci. USA, 99, 4349–4354.
Tsihlias J, Zhang W, Bhattacharya N, Flanagan M, Klotz L and Slingerland J . (2000). Oncogene, 19, 670–679.
Zhao XY, Malloy PJ, Krishnan AV, Swami S, Navone NM, Peehl DM and Feldman D . (2000). Nat. Med., 6, 703–706.
Acknowledgements
This work was supported by the Claire and John Bertucci Prostate Cancer Research Fund. We acknowledge the kind generosity of Dr Dario Altieri (University of Massachusetts Medical Center) for providing the following adenoviral constructs: pAd-S(T34A) and pAd-S(WT), and Dr A Bellacosa (Fox Chase Cancer Center) for providing us with the AKT-KD constructs described in the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, M., Latham, D., Delaney, M. et al. Survivin mediates resistance to antiandrogen therapy in prostate cancer. Oncogene 24, 2474–2482 (2005). https://doi.org/10.1038/sj.onc.1208490
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1208490
Keywords
This article is cited by
-
Cloning, Expression, and Purification of the Human Synthetic Survivin Protein in Escherichia coli Using Response Surface Methodology (RSM)
Molecular Biotechnology (2023)
-
Subcellular Compartmentalization of Survivin is Associated with Biological Aggressiveness and Prognosis in Prostate Cancer
Scientific Reports (2020)
-
Gemigliptin, a novel dipeptidyl peptidase-IV inhibitor, exerts a synergistic cytotoxicity with the histone deacetylase inhibitor PXD101 in thyroid carcinoma cells
Journal of Endocrinological Investigation (2018)
-
Survivin, a molecular target for therapeutic interventions in squamous cell carcinoma
Cellular & Molecular Biology Letters (2017)
-
Co-targeting EGFR and survivin with a bivalent aptamer-dual siRNA chimera effectively suppresses prostate cancer
Scientific Reports (2016)