Abstract
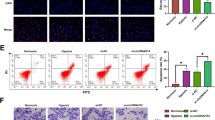
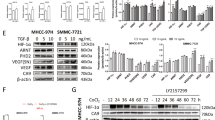
Hepatobiliary and pancreatic carcinomas are hypovascular tumors that can proliferate under hypoxic conditions. Recent reports have demonstrated that hypoxia-inducible factor 1 alpha (HIF1α) plays an important role in the survival of these cancers. Given these findings, the inhibition of the HIF1α pathway might prove to be a powerful tool in the treatment of these cancers. To inhibit HIF1α expression, we used small interference RNA (siRNA) expression vectors in this study. The transient transfection of siRNA expression vectors significantly reduced both HIF1α mRNA levels (13% of control) and protein levels (41% of control) and significantly inhibited the growth of cancer cell lines (P<0.05). VEGF, Glut1, and aldorase A expressions were also significantly reduced by transfection with these vectors (P<0.05), and we found that these vectors induced apoptosis but not cell cycle arrest. In a subcutaneous tumor model using nude mice, transfected MIA PaCa-2 cells, stably expressing siRNAs, barely formed tumors compared to control (P<0.05). This study thus demonstrates the usefulness of siRNA expression vector in targeting HIF1α and points to a potential clinical role in the treatment of pancreatic and hepatobiliary carcinomas.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Wang GL, Jiang BH, Rue EA, Semenza GL . Hypoxia-inducible factor 1 is a basic–helix–loop–helix–PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci USA 1995; 92: 5510–5514.
Masson N, Willam C, Maxwell PH, Pugh CW, Ratcliffe PJ . Independent function of two destruction domains in hypoxia-inducible factor-alpha chains activated by prolyl hydroxylation. EMBO J 2001; 20: 5197–5206.
Pugh CW, Ratcliffe PJ . Regulation of angiogenesis by hypoxia: role of the HIF system. Nat Med 2003; 9: 677–684.
Shibaji T, Nagao M, Ikeda N, Kanehiro H, Hisanaga M, Ko S et al. Prognostic significance of HIF-1 alpha overexpression in human pancreatic cancer. Anticancer Res 2003; 23: 4721–4727.
Kurokawa T, Miyamoto M, Kato K, Cho Y, Kawarada Y, Hida Y et al. Overexpression of hypoxia-inducible-factor 1alpha(HIF-1alpha) in oesophageal squamous cell carcinoma correlates with lymph node metastasis and pathologic stage. Br J Cancer 2003; 89: 1042–1047.
Buchler P, Reber HA, Buchler M, Shrinkante S, Buchler MW, Friess H et al. Hypoxia-inducible factor 1 regulates vascular endothelial growth factor expression in human pancreatic cancer. Pancreas 2003; 26: 56–64.
Schindl M, Schoppmann SF, Samonigg H, Hausmaninger H, Kwasny W, Gnant M et al. Overexpression of hypoxia-inducible factor 1alpha is associated with an unfavorable prognosis in lymph node-positive breast cancer. Clin Cancer Res 2002; 8: 1831–1837.
Sun X, Kanwar JR, Leung E, Lehnert K, Wang D, Krissansen GW . Gene transfer of antisense hypoxia inducible factor-1 alpha enhances the therapeutic efficacy of cancer immunotherapy. Gene Therapy 2001; 8: 638–645.
Maemura K, Hsieh CM, Jain MK, Fukumoto S, Layne MD, Liu Y et al. Generation of a dominant-negative mutant of endothelial PAS domain protein 1 by deletion of a potent C-terminal transactivation domain. J Biol Chem 1999; 274: 31565–31570.
Rose F, Grimminger F, Appel J, Heller M, Pies V, Weissmann N et al. Hypoxic pulmonary artery fibroblasts trigger proliferation of vascular smooth muscle cells: role of hypoxia-inducible transcription factors. FASEB J 2002; 16: 1660–1661.
Hunter T, Hunt T, Jackson RJ, Robertson HD . The characteristics of inhibition of protein synthesis by double-stranded ribonucleic acid in reticulocyte lysates. J Biol Chem 1975; 250: 409–417.
Elbashir SM, Harborth J, Lendeckel W, Yalcin A, Weber K, Tuschl T . Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature 2001; 411: 494–498.
Miyagishi M, Hayashi M, Taira K . Comparison of the suppressive effects of antisense oligonucleotides and siRNAs directed against the same targets in mammalian cells. Antisense Nucleic Acid Drug Dev 2003; 13: 1–7.
Miyagishi M, Taira K . U6 promoter-driven siRNAs with four uridine 3′ overhangs efficiently suppress targeted gene expression in mammalian cells. Nat Biotechnol 2002; 20: 497–500.
Brummelkamp TR, Bernards R, Agami R . A system for stable expression of short interfering RNAs in mammalian cells. Science 2002; 296: 550–553.
Lee NS, Dohjima T, Bauer G, Li H, Li MJ, Ehsani A et al. Expression of small interfering RNAs targeted against HIV-1 rev transcripts in human cells. Nat Biotechnol 2002; 20: 500–505.
Paul CP, Good PD, Winer I, Engelke DR . Effective expression of small interfering RNA in human cells. Nat Biotechnol 2002; 20: 505–508.
Paddison PJ, Caudy AA, Bernstein E, Hannon GJ, Conklin DS . Short hairpin RNAs (shRNAs) induce sequence-specific silencing in mammalian cells. Genes Dev 2002; 16: 948–958.
Sui G, Soohoo C, Affarel B, Gay F, Shi Y, Forrester WC . A DNA vector-based RNAi technology to suppress gene expression in mammalian cells. Proc Natl Acad Sci USA 2002; 99: 5515–5520.
Yu JY, DeRuiter SL, Turner DL . RNA interference by expression of short-interfering RNAs and hairpin RNAs in mammalian cells. Proc Natl Acad Sci USA 2002; 99: 6047–6052.
McManus MT, Petersen CP, Haines BB, Chen J, Sharp PA . Gene silencing using micro-RNA designed hairpins. RNA 2002; 8: 842–850.
Elbashir SM, Lendeckel W, Tuschl T . RNA interference is mediated by 21- and 22-nucleotide RNAs. Genes Dev 2001; 15: 188–200.
Bernstein E, Caudy AA, Hammond SM, Hannon GJ . Role for a bidentate ribonuclease in the initiation step of RNA interference. Nature 2001; 409: 363–366.
Jackson AL, Bartz SR, Schelter J, Kobayashi SV, Burchard J, Mao M et al. Expression profiling reveals off-target gene regulation by RNAi. Nat Biotechnol 2003; 21: 635–637.
Talks KL, Turley H, Gatter KC, Maxwell PH, Pugh CW, Ratcliffe PJ et al. The expression and distribution of the hypoxia-inducible factors HIF-1alpha and HIF-2alpha in normal human tissues, cancers, and tumor-associated macrophages. Am J Pathol 2000; 157: 411–421.
Zhong H, De Marzo AM, Laughner E, Lim M, Holton DA, Zagzag D et al. Overexpression of hypoxia-inducible factor 1alpha in common human cancers and their metastases. Cancer Res 1999; 59: 5830–5835.
Stebbins CE, Kaelin Jr WG, Pavletich NP . Structure of the VHL–ElonginC–ElonginB complex: implications for VHL tumor suppressor function. Science 1999; 284: 455–461.
Tanimoto K, Makino Y, Pereira T, Poellinger L . Mechanism of regulation of the hypoxia-inducible factor-1 alpha by the von Hippel–Lindau tumor suppressor protein. EMBO J 2000; 19: 4298–4309.
Clifford SC, Astuti D, Hooper L, Maxwell PH, Ratcliffe PJ, Maher ER . The pVHL-associated SCF ubiquitin ligase complex: molecular genetic analysis of elongin B and C, Rbx1 and HIF-1alpha in renal cell carcinoma. Oncogene 2001; 20: 5067–5074.
Graeber TG, Osmanian C, Jacks T, Housman DE, Koch CJ, Lowe SW et al. Hypoxia-mediated selection of cells with diminished apoptotic potential in solid tumours. Nature 1996; 379: 88–91.
Amellem O, Sandvik JA, Stokke T, Pettersen EO . The retinoblastoma protein-associated cell cycle arrest in S-phase under moderate hypoxia is disrupted in cells expressing HPV18 E7 oncoprotein. Br J Cancer 1998; 77: 862–872.
Schmaltz C, Hardenbergh PH, Wells A, Fisher DE . Regulation of proliferation–survival decisions during tumor cell hypoxia. Mol Cell Biol 1998; 18: 2845–2854.
Leuenroth SJ, Grutkoski PS, Ayala A, Simms HH . Suppression of PMN apoptosis by hypoxia is dependent on Mcl-1 and MAPK activity. Surgery 2000; 128: 171–177.
Hruban RH, Lacobuzio-Donahue C, Wilentz RE, Goggins M, Kern SE . Molecular pathology of pancreatic cancer. Cancer J 2001; 7: 251–258.
Butz J, Wickstrom E, Edwards J . Characterization of mutations and loss of heterozygosity of p53 and K-ras2 in pancreatic cancer cell lines by immobilized polymerase chain reaction. BMC Biotechnol 2003; 3: 11.
Ryan HE, Poloni M, McNulty W, Elson D, Gassmann M, Arbeit JM et al. Hypoxia-inducible factor-1alpha is a positive factor in solid tumor growth. Cancer Res 2000; 60: 4010–4015.
Baek JH, Jang JE, Kang CM, Chung HY, Kim ND, Kim KW . Hypoxia-induced VEGF enhances tumor survivability via suppression of serum deprivation-induced apoptosis. Oncogene 2000; 19: 4621–4631.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mizuno, T., Nagao, M., Yamada, Y. et al. Small interfering RNA expression vector targeting hypoxia-inducible factor 1 alpha inhibits tumor growth in hepatobiliary and pancreatic cancers. Cancer Gene Ther 13, 131–140 (2006). https://doi.org/10.1038/sj.cgt.7700871
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.cgt.7700871
Keywords
This article is cited by
-
DLK1: A Novel Target for Immunotherapeutic Remodeling of the Tumor Blood Vasculature
Molecular Therapy (2013)
-
Epigenetic regulation of hypoxia inducible factor in diseases and therapeutics
Archives of Pharmacal Research (2013)
-
RNA interference of hypoxia-inducible factor-1 alpha improves the effects of transcatheter arterial embolization in rat liver tumors
Tumor Biology (2012)
-
Silencing of HIF-1α suppresses tumorigenicity of renal cell carcinoma through induction of apoptosis
Cancer Gene Therapy (2010)
-
Se-methylselenocysteine sensitizes hypoxic tumor cells to irinotecan by targeting hypoxia-inducible factor 1α
Cancer Chemotherapy and Pharmacology (2010)