Abstract
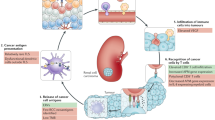
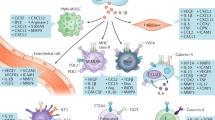
Kidney cancer has unique features that make this malignancy attractive for therapeutic approaches that target components of the immune system. Immune checkpoint inhibition is a well-established part of kidney cancer treatment, and rapid advances continue to be made in this field. Initial preclinical studies that elucidated the biology of the programmed cell death 1 (PD-1), programmed cell death 1 ligand 1 (PD-L1) and cytotoxic T lymphocyte antigen 4 (CTLA-4) immune checkpoints led to a series of clinical trials that resulted in regulatory approval of nivolumab and the combination of ipilimumab plus nivolumab for the treatment of advanced renal cell carcinoma. Subsequent data led to approvals of combination strategies of immune checkpoint inhibition plus agents that target the vascular endothelial growth factor receptor and a shift in the current standard of renal cell carcinoma care. However, controversies remain regarding the optimal therapy selection and treatment strategy for individual patients, which might be eventually overcome by current intensive efforts in biomarker research. That work includes evaluation of tumour cell PD-L1 expression, gene expression signatures, CD8+ T cell density and others. In the future, further advances in the understanding of immune checkpoint biology might reveal new therapeutic targets beyond PD-1, PD-L1 and CTLA-4, as well as new combination approaches.
Key points
-
Renal cell carcinoma (RCC) has unique characteristics that potentially increase its susceptibility to treatments that target components of the immune system, including immune checkpoint inhibition (ICI).
-
Preclinical and clinical studies have provided a rationale for the treatment of RCC with programmed cell death 1 (PD-1), programmed cell death 1 ligand 1 (PD-L1) and cytotoxic T lymphocyte antigen 4 (CTLA-4) blockade.
-
Combination regimens that include ICI are the current standard of care in advanced RCC, but controversies remain and a thoughtful and systematic approach to the selection of first-line therapy is needed.
-
Research into useful biomarkers for patient selection is ongoing and includes the evaluation of tumour cell PD-L1 expression, gene expression signatures, CD8+ T cell density and others.
-
Further developments in ICI in RCC will be driven by an improved understanding of the immunobiology of their efficacy and emerging biomarkers, which is also informed by findings in other malignancies.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Bray, F. et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 68, 394–424 (2018).
Ferlay J. et al. Cancer Today (Powered by GLOBOCAN 2018): IARC CancerBase No. 15. IARC https://publications.iarc.fr/Databases/Iarc-Cancerbases/Cancer-Today-Powered-By-GLOBOCAN-2018--2018 (2018).
National Cancer Institute. Cancer stat facts: kidney and renal pelvis cancer. SEER https://seer.cancer.gov/statfacts/html/kidrp.html (2009).
Ebele, J., Sauter, G., Epstein, J. & Sesterhenn, I (eds) Pathology and Genetics of Tumours of the Urinary System and Male Genital Organs. (International Agency for Research on Cancer, 2004).
Rini, B. I. et al. Atezolizumab plus bevacizumab versus sunitinib in patients with previously untreated metastatic renal cell carcinoma (IMmotion151): a multicentre, open-label, phase 3, randomised controlled trial. Lancet 393, 2404–2415 (2019).
Choueiri, T. K. et al. A phase 3, randomized, open-label study of nivolumab combined with cabozantinib vs sunitinib in patients with previously untreated advanced or metastatic renal cell carcinoma (RCC; CheckMate 9ER). J. Clin. Oncol. 36, TPS4598–TPS4598 (2018).
Motzer, R. J. et al. Avelumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N. Engl. J. Med. 380, 1103–1115 (2019).
Rini, B. I. et al. Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N. Engl. J. Med. 380, 1116–1127 (2019).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03138512 (2020).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03142334 (2019).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03024996 (2019).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03288532 (2018).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03055013 (2020).
Mantia, C. M. & McDermott, D. F. Vascular endothelial growth factor and programmed death-1 pathway inhibitors in renal cell carcinoma. Cancer 125, 4148–4157 (2019).
Jawanda, G. G. & Drachenberg, D. Spontaneous regression of biopsy proven primary renal cell carcinoma: a case study. Can. Urol. Assoc. J. 6, E203–E205 (2012).
Flanigan, R. C. et al. Nephrectomy followed by interferon alfa-2b compared with interferon alfa-2b alone for metastatic renal-cell cancer. N. Engl. J. Med. 345, 1655–1659 (2001).
Siska, P. J. et al. Mitochondrial dysregulation and glycolytic insufficiency functionally impair CD8 T cells infiltrating human renal cell carcinoma. JCI Insight 2, e93411 (2017).
Giraldo, N. A. et al. Orchestration and prognostic significance of immune checkpoints in the microenvironment of primary and metastatic renal cell cancer. Clin. Cancer Res. 21, 3031–3040 (2015).
Fridman, W. H., Pagès, F., Sautès-Fridman, C. & Galon, J. The immune contexture in human tumours: impact on clinical outcome. Nat. Rev. Cancer 12, 298–306 (2012).
Turajlic, S. et al. Insertion-and-deletion-derived tumour-specific neoantigens and the immunogenic phenotype: a pan-cancer analysis. Lancet Oncol. 18, 1009–1021 (2017).
Kandalaft, L. E., Motz, G. T., Busch, J. & Coukos, G. Angiogenesis and the tumor vasculature as antitumor immune modulators: the role of vascular endothelial growth factor and endothelin. Curr. Top. Microbiol. Immunol. 344, 129–148 (2011).
Nanus, D. M., Pfeffer, L. M., Bander, N. H., Bahri, S. & Albino, A. P. Antiproliferative and antitumor effects of alpha-interferon in renal cell carcinomas: correlation with the expression of a kidney-associated differentiation glycoprotein. Cancer Res. 50, 4190–4194 (1990).
Hervas-Stubbs, S. et al. Direct effects of type I interferons on cells of the immune system. Clin. Cancer Res. 17, 2619–2627 (2011).
Minasian, L. M. et al. Interferon alfa-2a in advanced renal cell carcinoma: treatment results and survival in 159 patients with long-term follow-up. J. Clin. Oncol. 11, 1368–1375 (1993).
Fyfe, G. et al. Results of treatment of 255 patients with metastatic renal cell carcinoma who received high-dose recombinant interleukin-2 therapy. J. Clin. Oncol. 13, 688–696 (1995).
McDermott, D. F. et al. Randomized phase III trial of high-dose interleukin-2 versus subcutaneous interleukin-2 and interferon in patients with metastatic renal cell carcinoma. J. Clin. Oncol. 23, 133–141 (2005).
Gleave, M. E. et al. Interferon gamma-1b compared with placebo in metastatic renal-cell carcinoma. Canadian Urologic Oncology Group. N. Engl. J. Med. 338, 1265–1271 (1998).
Childs, R. et al. Regression of metastatic renal-cell carcinoma after nonmyeloablative allogeneic peripheral-blood stem-cell transplantation. N. Engl. J. Med. 343, 750–758 (2000).
Avigan, D. Dendritic cell-tumor fusion vaccines for renal cell carcinoma. Clin. Cancer Res. 10, 6347S–6352S (2004).
Matsushita, H. et al. A pilot study of autologous tumor lysate-loaded dendritic cell vaccination combined with sunitinib for metastatic renal cell carcinoma. J. Immunother. Cancer 2, 30 (2014).
Ribas, A. & Wolchok, J. D. Cancer immunotherapy using checkpoint blockade. Science 359, 1350–1355 (2018).
Tivol, E. A. et al. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity 3, 541–547 (1995).
Freeman, G. J. et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J. Exp. Med. 192, 1027–1034 (2000).
Topalian, S. L. et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N. Engl. J. Med. 366, 2443–2454 (2012).
Schildberg, F. A., Klein, S. R., Freeman, G. J. & Sharpe, A. H. Coinhibitory pathways in the B7-CD28 ligand-receptor family. Immunity 44, 955–972 (2016).
Topalian, S. L., Drake, C. G. & Pardoll, D. M. Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell 27, 450–461 (2015).
Chen, L. et al. Costimulation of antitumor immunity by the B7 counterreceptor for the T lymphocyte molecules CD28 and CTLA-4. Cell 71, 1093–1102 (1992).
Leach, D. R., Krummel, M. F. & Allison, J. P. Enhancement of antitumor immunity by CTLA-4 blockade. Science 271, 1734–1736 (1996).
Kwon, E. D. et al. Manipulation of T cell costimulatory and inhibitory signals for immunotherapy of prostate cancer. Proc. Natl Acad. Sci. USA 94, 8099–8103 (1997).
Dong, H., Zhu, G., Tamada, K. & Chen, L. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat. Med. 5, 1365–1369 (1999).
Barber, D. L. et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature 439, 682–687 (2006).
Dong, H. et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat. Med. 8, 793–800 (2002).
Iwai, Y., Terawaki, S. & Honjo, T. PD-1 blockade inhibits hematogenous spread of poorly immunogenic tumor cells by enhanced recruitment of effector T cells. Int. Immunol. 17, 133–144 (2005).
Drake, C. G. & Stein, M. N. The immunobiology of kidney cancer. J. Clin. Oncol. 36, 3547–3552 (2018).
Patsoukis, N. et al. Selective effects of PD-1 on Akt and Ras pathways regulate molecular components of the cell cycle and inhibit T cell proliferation. Sci. Signal. 5, ra46 (2012).
Thompson, R. H. et al. Tumor B7-H1 is associated with poor prognosis in renal cell carcinoma patients with long-term follow-up. Cancer Res. 66, 3381–3385 (2006).
Thompson, R. H. et al. Costimulatory B7-H1 in renal cell carcinoma patients: indicator of tumor aggressiveness and potential therapeutic target. Proc. Natl Acad. Sci. USA 101, 17174–17179 (2004).
Iacovelli, R. et al. Prognostic role of PD-L1 expression in renal cell carcinoma. a systematic review and meta-analysis. Target. Oncol. 11, 143–148 (2016).
Yang, J. C. et al. Ipilimumab (anti-CTLA4 antibody) causes regression of metastatic renal cell cancer associated with enteritis and hypophysitis. J. Immunother. 30, 825–830 (2007).
Choueiri, T. K. et al. Immunomodulatory activity of nivolumab in metastatic renal cell carcinoma. Clin. Cancer Res. 22, 5461–5471 (2016).
Motzer, R. J. et al. Nivolumab for metastatic renal cell carcinoma: results of a randomized phase II trial. J. Clin. Oncol. 33, 1430–1437 (2015).
Motzer, R. J. et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N. Engl. J. Med. 373, 1803–1813 (2015).
McDermott, D. F. et al. Pembrolizumab monotherapy as first-line therapy in advanced clear cell renal cell carcinoma: results from cohort A of KEYNOTE-427. J. Clin. Oncol. 36 (Suppl. 15), 4500 (2018).
McDermott, D. F. et al. First-line pembrolizumab (pembro) monotherapy for advanced non-clear cell renal cell carcinoma (nccRCC): results from KEYNOTE-427 cohort B. J. Clin. Oncol. 37 (Suppl. 7), 546 (2019).
McDermott, D. F. et al. Atezolizumab, an anti-programmed death-ligand 1 antibody, in metastatic renal cell carcinoma: long-term safety, clinical activity, and immune correlates from a phase Ia study. J. Clin. Oncol. 34, 833–842 (2016).
McDermott, D. F. et al. Clinical activity and molecular correlates of response to atezolizumab alone or in combination with bevacizumab versus sunitinib in renal cell carcinoma. Nat. Med. 24, 749–757 (2018).
Friedman, C. F., Proverbs-Singh, T. A. & Postow, M. A. Treatment of the immune-related adverse effects of immune checkpoint inhibitors: a review. JAMA Oncol. 2, 1346 (2016).
Curran, M. A., Montalvo, W., Yagita, H. & Allison, J. P. PD-1 and CTLA-4 combination blockade expands infiltrating T cells and reduces regulatory T and myeloid cells within B16 melanoma tumors. Proc. Natl Acad. Sci. USA 107, 4275–4280 (2010).
Selby, M. J. et al. Preclinical development of ipilimumab and nivolumab combination immunotherapy: mouse tumor models, in vitro functional studies, and cynomolgus macaque toxicology. PLoS One 11, e0161779 (2016).
Wolchok, J. D. et al. Nivolumab plus ipilimumab in advanced melanoma. N. Engl. J. Med. 369, 122–133 (2013).
Hammers, H. J. et al. Safety and efficacy of nivolumab in combination with ipilimumab in metastatic renal cell carcinoma: the checkmate 016 study. J. Clin. Oncol. 35, 3851–3858 (2017).
Motzer, R. J. et al. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N. Engl. J. Med. 378, 1277–1290 (2018).
Motzer, R. J. et al. Nivolumab plus ipilimumab versus sunitinib in first-line treatment for advanced renal cell carcinoma: extended follow-up of efficacy and safety results from a randomised, controlled, phase 3 trial. Lancet Oncol. 20, 1370–1385 (2019).
Tannir, N. M. et al. Thirty-month follow-up of the phase III checkmate 214 trial of first-line nivolumab + ipilimumab (N+I) or sunitinib (S) in patients (pts) with advanced renal cell carcinoma (aRCC). J. Clin. Oncol. 37 (Suppl. 7), 547 (2019).
Cella, D. et al. Patient-reported outcomes of patients with advanced renal cell carcinoma treated with nivolumab plus ipilimumab versus sunitinib (CheckMate 214): a randomised, phase 3 trial. Lancet Oncol. 20, 297–310 (2019).
National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology, Kidney Cancer (Version 2.2020). NCCN https://www.nccn.org/professionals/physician_gls/pdf/kidney.pdf (2020).
Schaubel, D. E. & Wei, G. Double inverse-weighted estimation of cumulative treatment effects under nonproportional hazards and dependent censoring. Biometrics 67, 29–38 (2011).
Gossage, L., Eisen, T. & Maher, E. R. VHL, the story of a tumour suppressor gene. Nat. Rev. Cancer 15, 55–64 (2015).
Hegde, P. S., Wallin, J. J. & Mancao, C. Predictive markers of anti-VEGF and emerging role of angiogenesis inhibitors as immunotherapeutics. Semin. Cancer Biol. 52, 117–124 (2018).
Jain, R. K. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science 307, 58–62 (2005).
Nair, S. et al. Synergy between tumor immunotherapy and antiangiogenic therapy. Blood 102, 964–971 (2003).
Manning, E. A. et al. A vascular endothelial growth factor receptor-2 inhibitor enhances antitumor immunity through an immune-based mechanism. Clin. Cancer Res. 13, 3951–3959 (2007).
Shrimali, R. K. et al. Antiangiogenic agents can increase lymphocyte infiltration into tumor and enhance the effectiveness of adoptive immunotherapy of cancer. Cancer Res. 70, 6171–6180 (2010).
Elamin, Y. Y., Rafee, S., Toomey, S. & Hennessy, B. T. Immune effects of bevacizumab: killing two birds with one stone. Cancer Microenviron. 8, 15–21 (2015).
Huang, Y. et al. Vascular normalizing doses of antiangiogenic treatment reprogram the immunosuppressive tumor microenvironment and enhance immunotherapy. Proc. Natl Acad. Sci. USA 109, 17561–17566 (2012).
Henze, A.-T. & Mazzone, M. The impact of hypoxia on tumor-associated macrophages. J. Clin. Invest. 126, 3672–3679 (2016).
Schor-Bardach, R. et al. Does arterial spin-labeling MR imaging-measured tumor perfusion correlate with renal cell cancer response to antiangiogenic therapy in a mouse model? Radiology 251, 731–742 (2009).
Escudier, B. et al. Bevacizumab plus interferon alfa-2a for treatment of metastatic renal cell carcinoma: a randomised, double-blind phase III trial. Lancet 370, 2103–2111 (2007).
Rini, B. I. et al. Phase III trial of bevacizumab plus interferon alfa versus interferon alfa monotherapy in patients with metastatic renal cell carcinoma: final results of CALGB 90206. J. Clin. Oncol. 28, 2137–2143 (2010).
Wallin, J. J. et al. Atezolizumab in combination with bevacizumab enhances antigen-specific T-cell migration in metastatic renal cell carcinoma. Nat. Commun. 7, 12624 (2016).
Atkins, M. B. et al. IMmotion150: A phase II trial in untreated metastatic renal cell carcinoma (mRCC) patients (pts) of atezolizumab (atezo) and bevacizumab (bev) vs and following atezo or sunitinib (sun). J. Clin. Oncol. 35, 4505–4505 (2017).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02420821 (2019).
Barthelemy, P. et al. 947PTiNivo: tivozanib combined with nivolumab results in prolonged progression free survival in patients with metastatic renal cell carcinoma (mRCC): final results. Ann. Oncol. 30, mdz249.043 (2019).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03937219 (2019).
Grünwald, V. et al. Lenvatinib plus everolimus or pembrolizumab versus sunitinib in advanced renal cell carcinoma: study design and rationale. Future Oncol. 15, 929–941 (2019).
Ravaud, A. et al. Adjuvant sunitinib in high-risk renal-cell carcinoma after nephrectomy. N. Engl. J. Med. 375, 2246–2254 (2016).
Haas, N. B. et al. Adjuvant sunitinib or sorafenib for high-risk, non-metastatic renal-cell carcinoma (ECOG-ACRIN E2805): a double-blind, placebo-controlled, randomised, phase 3 trial. Lancet 387, 2008–2016 (2016).
Gross-Goupil, M. et al. Axitinib versus placebo as an adjuvant treatment of renal cell carcinoma: results from the phase III, randomized ATLAS trial. Ann. Oncol. 29, 2371–2378 (2018).
Motzer, R. J. et al. Randomized phase III trial of adjuvant pazopanib versus placebo after nephrectomy in patients with localized or locally advanced renal cell carcinoma. J. Clin. Oncol. 35, 3916–3923 (2017).
Eisen, T. et al. Primary efficacy analysis from the SORCE trial (RE05): adjuvant sorafenib for renal cell carcinoma at intermediate or high risk of relapse. Ann. Oncol. 30 (Suppl 5), v851–v934 (2019).
Eggermont, A. M. M. et al. Adjuvant ipilimumab versus placebo after complete resection of high-risk stage III melanoma (EORTC 18071): a randomised, double-blind, phase 3 trial. Lancet Oncol. 16, 522–530 (2015).
Eggermont, A. M. M. et al. Prolonged survival in stage III melanoma with ipilimumab adjuvant therapy. N. Engl. J. Med. 375, 1845–1855 (2016).
Eggermont, A. M. M. et al. Adjuvant pembrolizumab versus placebo in resected stage III melanoma. N. Engl. J. Med. 378, 1789–1801 (2018).
Weber, J. et al. Adjuvant nivolumab versus ipilimumab in resected stage III or IV melanoma. N. Engl. J. Med. 377, 1824–1835 (2017).
Holash, J. et al. Vessel cooption, regression, and growth in tumors mediated by angiopoietins and VEGF. Science 284, 1994–1998 (1999).
Liu, J. et al. Improved efficacy of neoadjuvant compared to adjuvant immunotherapy to eradicate metastatic disease. Cancer Discov. 6, 1382–1399 (2016).
Eskens, F. A. L. M. & Verweij, J. The clinical toxicity profile of vascular endothelial growth factor (VEGF) and vascular endothelial growth factor receptor (VEGFR) targeting angiogenesis inhibitors; a review. Eur. J. Cancer 42, 3127–3139 (2006).
Wolchok, J. D. et al. Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N. Engl. J. Med. 377, 1345–1356 (2017).
Antonia, S. J. et al. Nivolumab alone and nivolumab plus ipilimumab in recurrent small-cell lung cancer (CheckMate 032): a multicentre, open-label, phase 1/2 trial. Lancet Oncol. 17, 883–895 (2016).
Amos, S. M. et al. Autoimmunity associated with immunotherapy of cancer. Blood 118, 499–509 (2011).
Brahmer, J. R. et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology clinical practice guideline. J. Clin. Oncol. 36, 1714–1768 (2018).
Puzanov, I. et al. Managing toxicities associated with immune checkpoint inhibitors: consensus recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J. Immunother. Cancer 5, 95 (2017).
Borghaei, H. et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N. Engl. J. Med. 373, 1627–1639 (2015).
Paz-Ares, L. et al. Pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. N. Engl. J. Med. 379, 2040–2051 (2018).
Gandhi, L. et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N. Engl. J. Med. 378, 2078–2092 (2018).
Robert, C. et al. Nivolumab in previously untreated melanoma without BRAF mutation. N. Engl. J. Med. 372, 320–330 (2015).
Callea, M. et al. Differential expression of PD-L1 between primary and metastatic sites in clear-cell renal cell carcinoma. Cancer Immunol. Res. 3, 1158–1164 (2015).
Barsoum, I. B., Smallwood, C. A., Siemens, D. R. & Graham, C. H. A mechanism of hypoxia-mediated escape from adaptive immunity in cancer cells. Cancer Res. 74, 665–674 (2014).
Chen, S. et al. Mechanisms regulating PD-L1 expression on tumor and immune cells. J. Immunother. Cancer 7, 305 (2019).
Noguchi, T. et al. Temporally distinct PD-L1 expression by tumor and host cells contributes to immune escape. Cancer Immunol. Res. 5, 106–117 (2017).
Udall, M. et al. PD-L1 diagnostic tests: a systematic literature review of scoring algorithms and test-validation metrics. Diagn. Pathol. 13, 12 (2018).
Hansen, A. R. & Siu, L. L. PD-L1 testing in cancer: challenges in companion diagnostic development. JAMA Oncol. 2, 15 (2016).
Yearley, J. H. et al. PD-L2 expression in human tumors: relevance to anti-PD-1 therapy in cancer. Clin. Cancer Res. 23, 3158–3167 (2017).
Motzer, R. J., Powles, T., Atkins, M. B. & Rini, B. I. IMmotion151: a randomized phase III study of atezolizumab plus bevacizumab vs sunitinib in untreated metastatic renal cell carcinoma (mRCC). J. Clin. Oncol. 36, 578 (2018).
Rini, B. I. et al. Molecular correlates differentiate response to atezolizumab (atezo) + bevacizumab (bev) vs sunitinib (sun): results from a phase III study (IMmotion151) in untreated metastatic renal cell carcinoma (mRCC). Ann. Oncol. 29 (Suppl. 8), LBA31 (2018).
Choueiri, T. K. et al. Biomarker analyses from JAVELIN renal 101: avelumab + axitinib (A+Ax) versus sunitinib (S) in advanced renal cell carcinoma (aRCC). J. Clin. Oncol. 37, 101–101 (2019).
Rooney, M. S., Shukla, S. A., Wu, C. J., Getz, G. & Hacohen, N. Molecular and genetic properties of tumors associated with local immune cytolytic activity. Cell 160, 48–61 (2015).
Miao, D. et al. Genomic correlates of response to immune checkpoint therapies in clear cell renal cell carcinoma. Science 359, 801–806 (2018).
Pan, D. et al. A major chromatin regulator determines resistance of tumor cells to T cell-mediated killing. Science 359, 770–775 (2018).
Nargund, A. M. et al. The SWI/SNF protein PBRM1 restrains vhl-loss-driven clear cell renal cell carcinoma. Cell Rep. 18, 2893–2906 (2017).
Voss, M. H. et al. Integrated biomarker analysis for 412 renal cell cancer (RCC) patients (pts) treated on the phase 3 COMPARZ trial: correlating common mutation events in PBRM1 and BAP1 with angiogenesis expression signatures and outcomes on tyrosine kinase inhibitor (TKI) therapy. J. Clin. Oncol. 35, 4523–4523 (2017).
George, D. J. et al. Immune biomarkers predictive for disease-free survival with adjuvant sunitinib in high-risk locoregional renal cell carcinoma: from randomized phase III S-TRAC study. Clin. Cancer Res. 24, 1554–1561 (2018).
Loo, K. et al. Partially exhausted tumor-infiltrating lymphocytes predict response to combination immunotherapy. JCI Insight 2, e93433 (2017).
Bellucci, R. et al. Interferon-γ-induced activation of JAK1 and JAK2 suppresses tumor cell susceptibility to NK cells through upregulation of PD-L1 expression. Oncoimmunology 4, e1008824 (2015).
Ayers, M. et al. IFN-γ-related mRNA profile predicts clinical response to PD-1 blockade. J. Clin. Invest. 127, 2930–2940 (2017).
O’Donnell, P. H. et al. Biomarker findings and mature clinical results from KEYNOTE-052: first-line pembrolizumab (pembro) in cisplatin-ineligible advanced urothelial cancer (UC). J. Clin. Oncol. 35, 4502–4502 (2017).
Anderson, A. C., Joller, N. & Kuchroo, V. K. Lag-3, Tim-3, and TIGIT: co-inhibitory receptors with specialized functions in immune regulation. Immunity 44, 989–1004 (2016).
Ascierto, P. A. et al. Initial efficacy of anti-lymphocyte activation gene-3 (anti–LAG-3; BMS-986016) in combination with nivolumab (nivo) in pts with melanoma (MEL) previously treated with anti–PD-1/PD-L1 therapy. J. Clin. Oncol. 35, 9520–9520 (2017).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03470922 (2019).
Guillerey, C. et al. TIGIT immune checkpoint blockade restores CD8+ T-cell immunity against multiple myeloma. Blood 132, 1689–1694 (2018).
Solomon, B. L. & Garrido-Laguna, I. TIGIT: a novel immunotherapy target moving from bench to bedside. Cancer Immunol. Immunother. 67, 1659–1667 (2018).
Harding, J. J. et al. A phase Ia/Ib study of an anti-TIM-3 antibody (LY3321367) monotherapy or in combination with an anti-PD-L1 antibody (LY3300054): interim safety, efficacy, and pharmacokinetic findings in advanced cancers. J. Clin. Oncol. 37, 12–12 (2019).
Fan, X., Quezada, S. A., Sepulveda, M. A., Sharma, P. & Allison, J. P. Engagement of the ICOS pathway markedly enhances efficacy of CTLA-4 blockade in cancer immunotherapy. J. Exp. Med. 211, 715–725 (2014).
Carthon, B. C. et al. Preoperative CTLA-4 blockade: tolerability and immune monitoring in the setting of a presurgical clinical trial. Clin. Cancer Res. 16, 2861–2871 (2010).
Bentebibel, S.-E. et al. A first-in-human study and biomarker analysis of NKTR-214, a novel IL2Rβγ-biased cytokine, in patients with advanced or metastatic solid tumors. Cancer Discov. 9, 711–721 (2019).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03729245 (2020).
Wang, S. et al. Intratumoral injection of a CpG oligonucleotide reverts resistance to PD-1 blockade by expanding multifunctional CD8+ T cells. Proc. Natl. Acad. Sci. USA 113, E7240–E7249 (2016).
Prins, R. M. et al. The TLR-7 agonist, imiquimod, enhances dendritic cell survival and promotes tumor antigen-specific T cell priming: relation to central nervous system antitumor immunity. J. Immunol. 176, 157–164 (2006).
Dovedi, S. J. et al. Intravenous administration of the selective toll-like receptor 7 agonist DSR-29133 leads to anti-tumor efficacy in murine solid tumor models which can be potentiated by combination with fractionated radiotherapy. Oncotarget 7, 17035–17046 (2016).
Fu, J. et al. STING agonist formulated cancer vaccines can cure established tumors resistant to PD-1 blockade. Sci. Transl. Med. 7, 283ra52 (2015).
Villanueva, M. T. STINGing systemically. Nat. Rev. Drug. Discov. 18, 15 (2019).
Sivan, A. et al. Commensal bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science 350, 1084–1089 (2015).
Vétizou, M. et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science 350, 1079–1084 (2015).
Derosa, L. et al. Impact of antibiotics on outcome in patients with metastatic renal cell carcinoma treated with immune checkpoint inhibitors. J. Clin. Oncol. 35, 462–462 (2017).
Routy, B. et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science 359, 91–97 (2018).
Wersäll, P. J. et al. Regression of non-irradiated metastases after extracranial stereotactic radiotherapy in metastatic renal cell carcinoma. Acta Oncol. 45, 493–497 (2006).
Poleszczuk, J. T. et al. Abscopal benefits of localized radiotherapy depend on activated T-cell trafficking and distribution between metastatic lesions. Cancer Res. 76, 1009–1018 (2016).
Postow, M. A. et al. Immunologic correlates of the abscopal effect in a patient with melanoma. N. Engl. J. Med. 366, 925–931 (2012).
Lamers, C. H. J., Klaver, Y., Gratama, J. W., Sleijfer, S. & Debets, R. Treatment of metastatic renal cell carcinoma (mRCC) with CAIX CAR-engineered T-cells — a completed study overview. Biochem. Soc. Trans. 44, 951–959 (2016).
Chong, E. A. et al. PD-1 blockade modulates chimeric antigen receptor (CAR)-modified T cells: refueling the CAR. Blood 129, 1039–1041 (2017).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02811861 (2019).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03141177 (2019).
Author information
Authors and Affiliations
Contributions
W.X. researched data for the article. All authors made substantial contributions to discussion of the article content, and wrote and reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
M.B.A. serves on the advisory boards of Bristol-Myers Squibb (BMS), Merck, Novartis, Arrowhead, Pfizer, Galactone, Werewolf and Fathom. He serves as a consultant to BMS, Merck, Novartis, Pfizer, Genentech-Roche, Exelixis, Eisai, Aveo, Array, Ideera, Aduro, ImmunoCore, Boehringer-Ingelheim, Iovance, Newlink, Surface, Alexion, Acceleron, Pyxis, Cota and Amgen. He receives research support from BMS (to his institution) and owns stock options in Werewolf and Pyxis. D.F.M. receives consulting honoraria from BMS, Pfizer, Merck, Novartis, Exelixis, Array BioPharm, Genentech, Alkermes, Jounce Therapeutics, X4 Pharmaceuticals, Peloton, EMD Serono and Eli Lilly. He receives research support from BMS, Prometheus Laboratories, Merck, Genentech, Pfizer, Exelixis, Novartis, X4 Pharmaceuticals, Alkermes and Peloton. W.X. declares no competing interests.
Additional information
Peer review information
Nature Reviews Urology thanks V. Grünwald, M. Santoni and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Xu, W., Atkins, M.B. & McDermott, D.F. Checkpoint inhibitor immunotherapy in kidney cancer. Nat Rev Urol 17, 137–150 (2020). https://doi.org/10.1038/s41585-020-0282-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41585-020-0282-3
This article is cited by
-
Integration of basement membrane-related genes in a risk signature for prognosis in clear cell renal cell carcinoma
Scientific Reports (2024)
-
TRIB3 promotes the progression of renal cell carcinoma by upregulating the lipid droplet-associated protein PLIN2
Cell Death & Disease (2024)
-
Single-cell analysis reveals ADGRL4+ renal tubule cells as a highly aggressive cell type in clear cell renal cell carcinoma
Scientific Reports (2024)
-
Development of small-molecular-based radiotracers for PET imaging of PD-L1 expression and guiding the PD-L1 therapeutics
European Journal of Nuclear Medicine and Molecular Imaging (2024)
-
Selection of M7G-related lncRNAs in kidney renal clear cell carcinoma and their putative diagnostic and prognostic role
BMC Urology (2023)