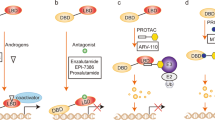
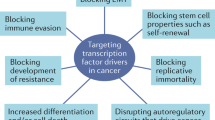
Abstract
Transcription factors (TFs) represent key biological players in diseases including cancer, autoimmunity, diabetes and cardiovascular disease. However, outside nuclear receptors, TFs have traditionally been considered ‘undruggable’ by small-molecule ligands due to significant structural disorder and lack of defined small-molecule binding pockets. Renewed interest in the field has been ignited by significant progress in chemical biology approaches to ligand discovery and optimization, especially the advent of targeted protein degradation approaches, along with increasing appreciation of the critical role a limited number of collaborators play in the regulation of key TF effector genes. Here, we review current understanding of TF-mediated gene regulation, discuss successful targeting strategies and highlight ongoing challenges and emerging approaches to address them.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Lambert, S. A. et al. The human transcription factors. Cell 172, 650–665 (2018).
Vernimmen, D. & Bickmore, W. A. The hierarchy of transcriptional activation: from enhancer to promoter. Trends Genet. 31, 696–708 (2015).
Lee, T. I. & Young, R. A. Transcriptional regulation and its misregulation in disease. Cell 152, 1237–1251 (2013). This review is a valuable reference for understanding the mechanisms by which transcription is dysregulated in disease.
Brivanlou, A. H. & Darnell, J. E. Signal transduction and the control of gene expression. Science 295, 813–818 (2002).
Darnell, J. E. Transcription factors as targets for cancer therapy. Nat. Rev. Cancer 2, 740–749 (2002).
Bushweller, J. H. Targeting transcription factors in cancer — from undruggable to reality. Nat. Rev. Cancer 19, 611–624 (2019).
Shah, D. R., Shah, R. R. & Morganroth, J. Tyrosine kinase inhibitors: their on-target toxicities as potential indicators of efficacy. Drug Saf. 36, 413–426 (2013).
Lehal, R. et al. Pharmacological disruption of the Notch transcription factor complex. Proc. Natl Acad. Sci. USA 117, 16292–16301 (2020). This paper demonstrates pharmacological advantages for directly targeting TFs over upstream signalling proteins and is one of the few studies that use functional target identification methods to support an on-target mechanism for an ‘undruggable’ TF inhibitor.
Lovly, C. M. & Shaw, A. T. Molecular pathways: resistance to kinase inhibitors and implications for therapeutic strategies. Clin. Cancer Res. 20, 2249–2256 (2014).
Gronemeyer, H., Gustafsson, J.-Å. & Laudet, V. Principles for modulation of the nuclear receptor superfamily. Nat. Rev. Drug Discov. 3, 950–964 (2004).
Liu, J. et al. Intrinsic disorder in transcription factors. Biochemistry 45, 6873–6888 (2006).
Voss, T. C. & Hager, G. L. Dynamic regulation of transcriptional states by chromatin and transcription factors. Nat. Rev. Genet. 15, 69–81 (2014). This article presents a comprehensive review of spatial and temporal mechanisms of transcriptional regulation, and highlights the importance of studying transcription from the perspective of single TF molecules.
Boija, A. et al. Transcription factors activate genes through the phase-separation capacity of their activation domains. Cell 175, 1842–1855.e16 (2018).
Ptashne, M. & Gann, A. Transcriptional activation by recruitment. Nature 386, 569–577 (1997).
Allen, B. L. & Taatjes, D. J. The Mediator complex: a central integrator of transcription. Nat. Rev. Mol. Cell Biol. 16, 155–166 (2015).
Centore, R. C., Sandoval, G. J., Soares, L. M. M., Kadoch, C. & Chan, H. M. Mammalian SWI/SNF chromatin remodeling complexes: emerging mechanisms and therapeutic strategies. Trends Genet. 36, 936–950 (2020).
Lee, T. I. & Young, R. A. Transcription of eukaryotic protein-coding genes. Annu. Rev. Genet. 34, 77–137 (2000).
Spitz, F. & Furlong, E. E. M. Transcription factors: from enhancer binding to developmental control. Nat. Rev. Genet. 13, 613–626 (2012).
Giacinti, C. & Giordano, A. RB and cell cycle progression. Oncogene 25, 5220–5227 (2006).
Rohs, R. et al. Origins of specificity in protein–DNA recognition. Annu. Rev. Biochem. 79, 233–269 (2010).
Goldstein, I. & Hager, G. L. Dynamic enhancer function in the chromatin context: dynamic mechanism for enhancer activation. Wiley Interdiscip. Rev. Syst. Biol. Med. 10, e1390 (2018).
Dames, S. A., Martinez-Yamout, M., De Guzman, R. N., Dyson, H. J. & Wright, P. E. Structural basis for Hif-1/CBP recognition in the cellular hypoxic response. Proc. Natl Acad. Sci. USA 99, 5271–5276 (2002).
Zor, T., De Guzman, R. N., Dyson, H. J. & Wright, P. E. Solution structure of the KIX domain of CBP bound to the transactivation domain of c-Myb. J. Mol. Biol. 337, 521–534 (2004).
Dyson, H. J. & Wright, P. E. Role of intrinsic protein disorder in the function and interactions of the transcriptional coactivators CREB-binding protein (CBP) and p300. J. Biol. Chem. 291, 6714–6722 (2016).
Ma, J. & Ptashne, M. A new class of yeast transcriptional activators. Cell 51, 113–119 (1987).
Warfield, L., Tuttle, L. M., Pacheco, D., Klevit, R. E. & Hahn, S. A sequence-specific transcription activator motif and powerful synthetic variants that bind Mediator using a fuzzy protein interface. Proc. Natl Acad. Sci. USA 111, E3506–E3513 (2014).
Staller, M. V. et al. A high-throughput mutational scan of an intrinsically disordered acidic transcriptional activation domain. Cell Syst. 6, 444–455.e6 (2018).
Sigler, P. B. Acid blobs and negative noodles. Nature 333, 210–212 (1988).
Tuttle, L. M. et al. Gcn4-mediator specificity is mediated by a large and dynamic fuzzy protein–protein complex. Cell Rep. 22, 3251–3264 (2018).
Chong, S. et al. Imaging dynamic and selective low-complexity domain interactions that control gene transcription. Science 361, eaar2555 (2018).
Henley, M. J. et al. Unexpected specificity within dynamic transcriptional protein–protein complexes. Proc. Natl Acad. Sci. USA 117, 27346–27353 (2020).
McSwiggen, D. T. et al. Evidence for DNA-mediated nuclear compartmentalization distinct from phase separation. eLife 8, e47098 (2019).
Loh, C.-Y. et al. Signal transducer and activator of transcription (STATs) proteins in cancer and inflammation: functions and therapeutic implication. Front. Oncol. 9, 48 (2019).
McEwan, I. J. in The Nuclear Receptor Superfamily Vol. 1443 (ed. McEwan, I. J.) 3–9 (Humana, 2016).
Jones, S. An overview of the basic helix–loop–helix proteins. Genome Biol. 5, 226 (2004).
Voss, T. C. et al. Dynamic exchange at regulatory elements during chromatin remodeling underlies assisted loading mechanism. Cell 146, 544–554 (2011).
Sabari, B. R. et al. Coactivator condensation at super-enhancers links phase separation and gene control. Science 361, eaar3958 (2018). This study represents the first demonstration that super-enhancers can concentrate transcriptional apparatus into phase-separated condensates.
Szabo, Q., Bantignies, F. & Cavalli, G. Principles of genome folding into topologically associating domains. Sci. Adv. 5, eaaw1668 (2019).
Beagan, J. A. & Phillips-Cremins, J. E. On the existence and functionality of topologically associating domains. Nat. Genet. 52, 8–16 (2020).
Paakinaho, V. et al. Single-molecule analysis of steroid receptor and cofactor action in living cells. Nat. Commun. 8, 15896 (2017).
Schoenfelder, S. & Fraser, P. Long-range enhancer–promoter contacts in gene expression control. Nat. Rev. Genet. 20, 437–455 (2019).
He, Y., Fang, J., Taatjes, D. J. & Nogales, E. Structural visualization of key steps in human transcription initiation. Nature 495, 481–486 (2013).
He, Y. et al. Near-atomic resolution visualization of human transcription promoter opening. Nature 533, 359–365 (2016).
Schilbach, S. et al. Structures of transcription pre-initiation complex with TFIIH and mediator. Nature 551, 204–209 (2017).
Bickmore, W. A. The spatial organization of the human genome. Annu. Rev. Genomics Hum. Genet. 14, 67–84 (2013).
Bonev, B. & Cavalli, G. Organization and function of the 3D genome. Nat. Rev. Genet. 17, 661–678 (2016).
Hnisz, D., Day, D. S. & Young, R. A. Insulated neighborhoods: structural and functional units of mammalian gene control. Cell 167, 1188–1200 (2016).
Dowen, J. M. et al. Control of cell identity genes occurs in insulated neighborhoods in mammalian chromosomes. Cell 159, 374–387 (2014).
Ghavi-Helm, Y. et al. Highly rearranged chromosomes reveal uncoupling between genome topology and gene expression. Nat. Genet. 51, 1272–1282 (2019).
Hnisz, D. et al. Super-enhancers in the control of cell identity and disease. Cell 155, 934–947 (2013).
Whyte, W. A. et al. Master transcription factors and mediator establish super-enhancers at key cell identity genes. Cell 153, 307–319 (2013).
Saint-André, V. et al. Models of human core transcriptional regulatory circuitries. Genome Res. 26, 385–396 (2016).
Hnisz, D. et al. Convergence of developmental and oncogenic signaling pathways at transcriptional super-enhancers. Mol. Cell 58, 362–370 (2015).
McNally, J. G. The glucocorticoid receptor: rapid exchange with regulatory sites in living cells. Science 287, 1262–1265 (2000).
Hager, G. L., McNally, J. G. & Misteli, T. Transcription dynamics. Mol. Cell 35, 741–753 (2009).
Lovén, J. et al. Selective inhibition of tumor oncogenes by disruption of super-enhancers. Cell 153, 320–334 (2013). This study is the first to show that super-enhancers can be more sensitive to inhibition of general transcriptional machinery than typical enhancers due to increased cooperativity of TFs and co-regulators.
Kwiatkowski, N. et al. Targeting transcription regulation in cancer with a covalent CDK7 inhibitor. Nature 511, 616–620 (2014).
Chipumuro, E. et al. CDK7 inhibition suppresses super-enhancer-linked oncogenic transcription in MYCN-driven cancer. Cell 159, 1126–1139 (2014). This paper shows that a covalent CDK7/12/13 inhibitor preferentially reduces MYCN-driven oncogenic transcriptional programmes by selectively targeting MYCN-associated super-enhancers.
Richters, A. et al. Modulating androgen receptor-driven transcription in prostate cancer with selective CDK9 inhibitors. Cell Chem. Biol. 28, 134–147.e14 (2021). This study identifies two potent and highly selective CDK9 inhibitors from a binding-focused screen of ‘undruggable’ ARV7 in cell lysates; similar to CDK7/12/13 inhibitors, these molecules show high selectivity for specific disease-related transcriptional programmes.
Gryder, B. E. et al. Chemical genomics reveals histone deacetylases are required for core regulatory transcription. Nat. Commun. 10, 3004 (2019).
Gryder, B. E. et al. Histone hyperacetylation disrupts core gene regulatory architecture in rhabdomyosarcoma. Nat. Genet. 51, 1714–1722 (2019).
Marques, J. G. et al. NuRD subunit CHD4 regulates super-enhancer accessibility in rhabdomyosarcoma and represents a general tumor dependency. eLife 9, e54993 (2020).
Wei, M.-T. et al. Nucleated transcriptional condensates amplify gene expression. Nat. Cell Biol. 22, 1187–1196 (2020).
Guo, Y. E. et al. Pol II phosphorylation regulates a switch between transcriptional and splicing condensates. Nature 572, 543–548 (2019).
Henninger, J. E. et al. RNA-mediated feedback control of transcriptional condensates. Cell 184, 207–225.e24 (2021).
Shrinivas, K. et al. Enhancer features that drive formation of transcriptional condensates. Mol. Cell 75, 549–561.e7 (2019).
Hnisz, D., Shrinivas, K., Young, R. A., Chakraborty, A. K. & Sharp, P. A. A phase separation model for transcriptional control. Cell 169, 13–23 (2017).
Li, W. et al. Biophysical properties of AKAP95 protein condensates regulate splicing and tumorigenesis. Nat. Cell Biol. 22, 960–972 (2020).
Plys, A. J. et al. Phase separation of Polycomb-repressive complex 1 is governed by a charged disordered region of CBX2. Genes Dev. 33, 799–813 (2019).
Gibson, B. A. et al. Organization of chromatin by intrinsic and regulated phase separation. Cell 179, 470–484.e21 (2019).
Sanulli, S. et al. HP1 reshapes nucleosome core to promote phase separation of heterochromatin. Nature 575, 390–394 (2019).
McSwiggen, D. T., Mir, M., Darzacq, X. & Tjian, R. Evaluating phase separation in live cells: diagnosis, caveats, and functional consequences. Genes Dev. 33, 1619–1634 (2019).
Mir, M., Bickmore, W., Furlong, E. E. M. & Narlikar, G. Chromatin topology, condensates and gene regulation: shifting paradigms or just a phase? Development 146, dev182766 (2019).
Bradner, J. E., Hnisz, D. & Young, R. A. Transcriptional addiction in cancer. Cell 168, 629–643 (2017).
Downward, J. Targeting RAS signalling pathways in cancer therapy. Nat. Rev. Cancer 3, 11–22 (2003).
O’Shea, J. J. et al. The JAK–STAT pathway: impact on human disease and therapeutic intervention. Annu. Rev. Med. 66, 311–328 (2015).
Taniguchi, K. & Karin, M. NF-κB, inflammation, immunity and cancer: coming of age. Nat. Rev. Immunol. 18, 309–324 (2018).
Kastenhuber, E. R. & Lowe, S. W. Putting p53 in context. Cell 170, 1062–1078 (2017).
Cardenas, M. G. et al. The expanding role of the BCL6 oncoprotein as a cancer therapeutic target. Clin. Cancer Res. 23, 885–893 (2017).
Dupain, C., Harttrampf, A. C., Urbinati, G., Geoerger, B. & Massaad-Massade, L. Relevance of fusion genes in pediatric cancers: toward precision medicine. Mol. Ther. Nucleic Acids 6, 315–326 (2017).
White, M. K., Pagano, J. S. & Khalili, K. Viruses and human cancers: a long road of discovery of molecular paradigms. Clin. Microbiol. Rev. 27, 463–481 (2014).
Flavahan, W. A. et al. Insulator dysfunction and oncogene activation in IDH mutant gliomas. Nature 529, 110–114 (2016).
Flavahan, W. A., Gaskell, E. & Bernstein, B. E. Epigenetic plasticity and the hallmarks of cancer. Science 357, eaal2380 (2017).
Ramsay, R. G. & Gonda, T. J. MYB function in normal and cancer cells. Nat. Rev. Cancer 8, 523–534 (2008).
Mansour, M. R. et al. An oncogenic super-enhancer formed through somatic mutation of a noncoding intergenic element. Science 346, 1373–1377 (2014).
Blackwell, T., Kretzner, L., Blackwood, E., Eisenman, R. & Weintraub, H. Sequence-specific DNA binding by the c-Myc protein. Science 250, 1149–1151 (1990).
Rahl, P. B. et al. c-Myc regulates transcriptional pause release. Cell 141, 432–445 (2010).
Rahl, P. B. & Young, R. A. MYC and transcription elongation. Cold Spring Harb. Perspect. Med. 4, a020990 (2014).
Lin, C. Y. et al. Transcriptional amplification in tumor cell elevated c-Myc. Cell 151, 56–67 (2012).
Schuijers, J. et al. Transcriptional dysregulation of MYC reveals common enhancer-docking mechanism. Cell Rep. 23, 349–360 (2018).
Felsher, D. W. & Bishop, J. M. Reversible tumorigenesis by MYC in hematopoietic lineages. Mol. Cell 4, 199–207 (1999).
Soucek, L. et al. Modelling Myc inhibition as a cancer therapy. Nature 455, 679–683 (2008).
Felsher, D. W. MYC inactivation elicits oncogene addiction through both tumor cell-intrinsic and host-dependent mechanisms. Genes Cancer 1, 597–604 (2010).
Tsherniak, A. et al. Defining a cancer dependency map. Cell 170, 564–576.e16 (2017).
Gryder, B. E. et al. PAX3–FOXO1 establishes myogenic super enhancers and confers BET bromodomain vulnerability. Cancer Discov. 7, 884–899 (2017).
Chapuy, B. et al. Discovery and characterization of super-enhancer-associated dependencies in diffuse large B cell lymphoma. Cancer Cell 24, 777–790 (2013).
Simmonds, R. E. & Foxwell, B. M. Signalling, inflammation and arthritis: NF-κB and its relevance to arthritis and inflammation. Rheumatology 47, 584–590 (2008).
Atreya, I., Atreya, R. & Neurath, M. F. NF-κB in inflammatory bowel disease. J. Intern. Med. 263, 591–596 (2008).
Leibowitz, S. M. & Yan, J. NF-κB pathways in the pathogenesis of multiple sclerosis and the therapeutic implications. Front. Mol. Neurosci. 9, 84 (2016).
Yue, Y., Stone, S. & Lin, W. Role of nuclear factor κB in multiple sclerosis and experimental autoimmune encephalomyelitis. Neural Regen. Res. 13, 1507 (2018).
Flanagan, S. E. et al. Activating germline mutations in STAT3 cause early-onset multi-organ autoimmune disease. Nat. Genet. 46, 812–814 (2014).
Milner, J. D. et al. Early-onset lymphoproliferation and autoimmunity caused by germline STAT3 gain-of-function mutations. Blood 125, 591–599 (2015).
Walford, H. H. & Doherty, T. A. STAT6 and lung inflammation. JAK-STAT 2, e25301 (2013).
Holland, S. M. et al. STAT3 mutations in the hyper-IgE syndrome. N. Engl. J. Med. 357, 1608–1619 (2007).
Skapenko, A., Leipe, J., Lipsky, P. E. & Schulze-Koops, H. The role of the T cell in autoimmune inflammation. Arthritis Res. Ther. 7, S4 (2005).
Ji, N., Sosa, R. A. & Forsthuber, T. G. More than just a T-box: the role of T-bet as a possible biomarker and therapeutic target in autoimmune diseases. Immunotherapy 3, 435–441 (2011).
Kanhere, A. et al. T-bet and GATA3 orchestrate TH1 and TH2 differentiation through lineage-specific targeting of distal regulatory elements. Nat. Commun. 3, 1268 (2012).
Capone, A. & Volpe, E. Transcriptional regulators of T helper 17 cell differentiation in health and autoimmune diseases. Front. Immunol. 11, 348 (2020).
Isono, F., Fujita-Sato, S. & Ito, S. Inhibiting RORγt/TH17 axis for autoimmune disorders. Drug Discov. Today 19, 1205–1211 (2014).
Bosnjak, B., Stelzmueller, B., Erb, K. J. & Epstein, M. M. Treatment of allergic asthma: modulation of TH2 cells and their responses. Respir. Res. 12, 114 (2011).
Fernando, V. et al. Regulation of an autoimmune model for multiple sclerosis in TH2-biased GATA3 transgenic mice. Int. J. Mol. Sci. 15, 1700–1718 (2014).
Tao, J.-H. et al. Foxp3, regulatory T cell, and autoimmune diseases. Inflammation 40, 328–339 (2017).
d’Hennezel, E. et al. FOXP3 forkhead domain mutation and regulatory T cells in the IPEX syndrome. N. Engl. J. Med. 361, 1710–1713 (2009).
Banerjee, S., Biehl, A., Gadina, M., Hasni, S. & Schwartz, D. M. JAK–STAT signaling as a target for inflammatory and autoimmune diseases: current and future prospects. Drugs 77, 521–546 (2017).
Udler, M. S., McCarthy, M. I., Florez, J. C. & Mahajan, A. Genetic risk scores for diabetes diagnosis and precision medicine. Endocr. Rev. 40, 1500–1520 (2019).
Pihoker, C. et al. Prevalence, characteristics and clinical diagnosis of maturity onset diabetes of the young due to mutations in HNF1A, HNF4A, and glucokinase: results from the SEARCH for diabetes in youth. J. Clin. Endocrinol. Metab. 98, 4055–4062 (2013).
Mitchell, S. M. S. & Frayling, T. M. The role of transcription factors in maturity-onset diabetes of the young. Mol. Genet. Metab. 77, 35–43 (2002).
Minra, N. & Tanaka, K. Analysis of the rat hepatocyte nuclear factor (HNF) 1 gene promoter: synergistic activation by HNF4 and HNF1 proteins. Nucleic Acids Res. 21, 3731–3736 (1993).
Grunert, M., Dorn, C. & Rickert-Sperling, S. in Congenital Heart Diseases: The Broken Heart (eds Rickert-Sperling, S., Kelly, R. G. & Driscoll, D. J.) 139–152 (Springer, 2016).
Kohli, S., Ahuja, S. & Rani, V. Transcription factors in heart: promising therapeutic targets in cardiac hypertrophy. Curr. Cardiol. Rev. 7, 262–271 (2012).
Epstein, J. A. & Buck, C. A. Transcriptional regulation of cardiac development: implications for congenital heart disease and DiGeorge syndrome. Pediatr. Res. 48, 717–724 (2000).
McCulley, D. J. & Black, B. L. in Current Topics in Developmental Biology Vol. 100 (ed. Bruneau, B. G.) 253–277 (Elsevier, 2012).
Hammoudeh, D. I., Follis, A. V., Prochownik, E. V. & Metallo, S. J. Multiple independent binding sites for small-molecule inhibitors on the oncoprotein c-Myc. J. Am. Chem. Soc. 131, 7390–7401 (2009). This study investigates the highly dynamic and non-specific binding modes of inhibitors of the disordered MYC TF; notably, the non-specific binding may be related to the promiscuous chemotypes of these molecules.
Baell, J. B. & Nissink, J. W. M. Seven year itch: pan-assay interference compounds (PAINS) in 2017—utility and limitations. ACS Chem. Biol. 13, 36–44 (2018).
Mazaira, G. I. et al. The nuclear receptor field: a historical overview and future challenges. Nucl. Recept. Res. 5, 101320 (2018).
Fernandez, E. J. Allosteric pathways in nuclear receptors — potential targets for drug design. Pharmacol. Ther. 183, 152–159 (2018).
de Vera, I. M. S. Advances in orphan nuclear receptor pharmacology: a new era in drug discovery. ACS Pharmacol. Transl. Sci. 1, 134–137 (2018).
Antonarakis, E. S. et al. AR-V7 and resistance to enzalutamide and abiraterone in prostate cancer. N. Engl. J. Med. 371, 1028–1038 (2014).
Zhu, Y. et al. Role of androgen receptor splice variant-7 (AR-V7) in prostate cancer resistance to 2nd-generation androgen receptor signaling inhibitors. Oncogene 39, 6935–6949 (2020).
Holden, J. K. & Cunningham, C. N. Targeting the Hippo pathway and cancer through the TEAD family of transcription factors. Cancers 10, 81 (2018).
Johnson, R. & Halder, G. The two faces of Hippo: targeting the Hippo pathway for regenerative medicine and cancer treatment. Nat. Rev. Drug Discov. 13, 63–79 (2014).
Dey, A., Varelas, X. & Guan, K.-L. Targeting the Hippo pathway in cancer, fibrosis, wound healing and regenerative medicine. Nat. Rev. Drug Discov. 19, 480–494 (2020).
Noland, C. L. et al. Palmitoylation of TEAD transcription factors is required for their stability and function in Hippo pathway signaling. Structure 24, 179–186 (2016).
Chan, P. et al. Autopalmitoylation of TEAD proteins regulates transcriptional output of the Hippo pathway. Nat. Chem. Biol. 12, 282–289 (2016).
Huh, H., Kim, D., Jeong, H.-S. & Park, H. Regulation of TEAD transcription factors in cancer biology. Cells 8, 600 (2019).
Pobbati, A. V. et al. Identification of quinolinols as activators of TEAD-dependent transcription. ACS Chem. Biol. 14, 2909–2921 (2019).
Bum-Erdene, K. et al. Small-molecule covalent modification of conserved cysteine leads to allosteric inhibition of the TEAD⋅Yap protein–protein interaction. Cell Chem. Biol. 26, 378–389.e13 (2019).
Holden, J. K. et al. Small molecule dysregulation of TEAD lipidation induces a dominant-negative inhibition of Hippo pathway signaling. Cell Rep. 31, 107809 (2020).
Kakiuchi-Kiyota, S., Schutten, M. M., Zhong, Y., Crawford, J. J. & Dey, A. Safety considerations in the development of Hippo pathway inhibitors in cancers. Front. Cell Dev. Biol. 7, 156 (2019).
Zhao, Y., Aguilar, A., Bernard, D. & Wang, S. Small-molecule inhibitors of the MDM2–p53 protein–protein interaction (MDM2 Inhibitors) in clinical trials for cancer treatment: miniperspective. J. Med. Chem. 58, 1038–1052 (2015).
Jones, S. N., Roe, A. E., Donehower, L. A. & Bradley, A. Rescue of embryonic lethality in Mdm2-deficient mice by absence of p53. Nature 378, 206–208 (1995).
Brown, C. J., Lain, S., Verma, C. S., Fersht, A. R. & Lane, D. P. Awakening guardian angels: drugging the p53 pathway. Nat. Rev. Cancer 9, 862–873 (2009).
Wells, M. et al. Structure of tumor suppressor p53 and its intrinsically disordered N-terminal transactivation domain. Proc. Natl Acad. Sci. USA 105, 5762–5767 (2008).
Estrada-Ortiz, N., Neochoritis, C. G. & Dömling, A. How to design a successful p53-MDM2/X interaction inhibitor: a thorough overview based on crystal structures. ChemMedChem 11, 757–772 (2016).
Chang, Y. S. et al. Stapled α-helical peptide drug development: a potent dual inhibitor of MDM2 and MDMX for p53-dependent cancer therapy. Proc. Natl Acad. Sci. USA 110, E3445–E3454 (2013).
Kaelin, W. G. Jr The von Hippel–Lindau tumour suppressor protein: O2 sensing and cancer. Nat. Rev. Cancer 8, 865–873 (2008).
Buckley, D. L. et al. Targeting the von Hippel–Lindau E3 ubiquitin ligase using small molecules to disrupt the VHL/HIF-1α interaction. J. Am. Chem. Soc. 134, 4465–4468 (2012). This paper is the first to demonstrate effective targeting of the TF HIF1α by the development of an inhibitor of the HIF1α-associated ubiquitin E3 ligase von Hippel–Lindau disease tumour suppressor.
Fraile, J. M., Quesada, V., Rodríguez, D., Freije, J. M. P. & López-Otín, C. Deubiquitinases in cancer: new functions and therapeutic options. Oncogene 31, 2373–2388 (2012).
Bruno, P. A., Morriss-Andrews, A., Henderson, A. R., Brooks, C. L. & Mapp, A. K. A synthetic loop replacement peptide that blocks canonical NF-κB signaling. Angew. Chem. Int. Ed. 55, 14997–15001 (2016).
Vincendeau, M. et al. Inhibition of canonical NF-κB signaling by a small molecule targeting NEMO–ubiquitin interaction. Sci. Rep. 6, 18934 (2016).
Maculins, T. et al. Discovery of protein–protein interaction inhibitors by integrating protein engineering and chemical screening platforms. Cell Chem. Biol. 27, 1441–1451.e7 (2020).
Miklossy, G., Hilliard, T. S. & Turkson, J. Therapeutic modulators of STAT signalling for human diseases. Nat. Rev. Drug Discov. 12, 611–629 (2013).
Rogers, J. L. et al. Development of inhibitors of the PAS-B domain of the HIF-2α transcription factor. J. Med. Chem. 56, 1739–1747 (2013).
Scheuermann, T. H. et al. Allosteric inhibition of hypoxia inducible factor-2 with small molecules. Nat. Chem. Biol. 9, 271–276 (2013). This paper describes the development and characterization of the first direct-binding allosteric inhibitor of the TF HIF2α.
Chen, W. et al. Targeting renal cell carcinoma with a HIF-2 antagonist. Nature 539, 112–117 (2016).
Wallace, E. M. et al. A small-molecule antagonist of HIF2α is efficacious in preclinical models of renal cell carcinoma. Cancer Res. 76, 5491–5500 (2016).
Xu, R. et al. 3-[(1S, 2S, 3R)-2,3-Difluoro-1-hydroxy-7-methylsulfonylindan-4-yl]oxy-5-fluorobenzonitrile (PT2977), a hypoxia-inducible factor 2α (HIF-2α) inhibitor for the treatment of clear cell renal cell carcinoma. J. Med. Chem. 62, 6876–6893 (2019).
Grembecka, J. et al. Menin–MLL inhibitors reverse oncogenic activity of MLL fusion proteins in leukemia. Nat. Chem. Biol. 8, 277–284 (2012). This paper describes the development and characterization of the first menin inhibitors that impede activity of the oncogenic TF MLL by blocking menin–MLL association.
Borkin, D. et al. Pharmacologic inhibition of the menin–MLL interaction blocks progression of MLL leukemia in vivo. Cancer Cell 27, 589–602 (2015).
Borkin, D. et al. Complexity of blocking bivalent protein–protein interactions: development of a highly potent inhibitor of the menin–mixed-lineage leukemia interaction. J. Med. Chem. 61, 4832–4850 (2018).
Klossowski, S. et al. Menin inhibitor MI-3454 induces remission in MLL1-rearranged and NPM1-mutated models of leukemia. J. Clin. Invest. 130, 981–997 (2020).
Xu, S. et al. Discovery of M-808 as a highly potent, covalent, small-molecule inhibitor of the menin–MLL interaction with strong in vivo antitumor activity. J. Med. Chem. 63, 4997–5010 (2020).
Kopan, R. & Ilagan, M. X. G. The canonical notch signaling pathway: unfolding the activation mechanism. Cell 137, 216–233 (2009).
Lukasik, S. M. et al. Altered affinity of CBFβ–SMMHC for Runx1 explains its role in leukemogenesis. Nat. Struct. Biol. 9, 674–679 (2002).
Illendula, A. et al. A small-molecule inhibitor of the aberrant transcription factor CBFβ–SMMHC delays leukemia in mice. Science 347, 779–784 (2015). This paper describes the first inhibitor of the oncogenic fusion CBFβ–SMMHC, which releases the RUNX1 TF from repressive CBFβ–SMMHC–RUNX1 complexes and delays progression of AML.
Struntz, N. B. et al. Stabilization of the Max homodimer with a small molecule attenuates Myc-driven transcription. Cell Chem. Biol. 26, 711–723.e14 (2019). This paper demonstrates that inhibition of the ‘undruggable’ TF MYC can be achieved by a small molecule that sequesters its requisite binding partner MAX into transcriptionally inactive homodimers.
Lao, B. B. et al. In vivo modulation of hypoxia-inducible signaling by topographical helix mimetics. Proc. Natl Acad. Sci. USA 111, 7531–7536 (2014).
Xie, X. et al. Targeting HPV16 E6-p300 interaction reactivates p53 and inhibits the tumorigenicity of HPV-positive head and neck squamous cell carcinoma. Oncogene 33, 1037–1046 (2014).
Wang, N. et al. Ordering a dynamic protein via a small-molecule stabilizer. J. Am. Chem. Soc. 135, 3363–3366 (2013).
Henderson, A. R. et al. Conservation of coactivator engagement mechanism enables small-molecule allosteric modulators. Proc. Natl Acad. Sci. USA 115, 8960–8965 (2018).
Cook, K. M. et al. Epidithiodiketopiperazines block the interaction between hypoxia-inducible factor-1α (HIF-1α) and p300 by a zinc ejection mechanism. J. Biol. Chem. 284, 26831–26838 (2009).
Majmudar, C. Y. et al. Sekikaic acid and lobaric acid target a dynamic interface of the coactivator CBP/p300. Angew. Chem. Int. Ed. 51, 11258–11262 (2012).
Ramaswamy, K. et al. Peptidomimetic blockade of MYB in acute myeloid leukemia. Nat. Commun. 9, 110 (2018).
Henchey, L. K. et al. Inhibition of hypoxia inducible factor 1—transcription coactivator interaction by a hydrogen bond surrogate α-Helix. J. Am. Chem. Soc. 132, 941–943 (2010).
Schreiber, S. L. The rise of molecular glues. Cell 184, 3–9 (2021).
Ito, T. et al. Identification of a primary target of thalidomide teratogenicity. Science 327, 1345–1350 (2010).
Kronke, J. et al. Lenalidomide causes selective degradation of IKZF1 and IKZF3 in multiple myeloma cells. Science 343, 301–305 (2014).
Lu, G. et al. The myeloma drug lenalidomide promotes the cereblon-dependent destruction of ikaros proteins. Science 343, 305–309 (2014). Together with Kronke et al. (2014), this study demonstrates that the anticancer IMiDs function by degrading the TFs IKZF1 and IKZF3.
Chamberlain, P. P. et al. Structure of the human cereblon–DDB1–lenalidomide complex reveals basis for responsiveness to thalidomide analogs. Nat. Struct. Mol. Biol. 21, 803–809 (2014).
Petzold, G., Fischer, E. S. & Thomä, N. H. Structural basis of lenalidomide-induced CK1α degradation by the CRL4CRBN ubiquitin ligase. Nature 532, 127–130 (2016).
Sievers, Q. L. et al. Defining the human C2H2 zinc finger degrome targeted by thalidomide analogs through CRBN. Science 362, eaat0572 (2018).
Isobe, Y. et al. Manumycin polyketides act as molecular glues between UBR7 and P53. Nat. Chem. Biol. 16, 1189–1198 (2020).
Hanan, E. J. et al. Monomeric targeted protein degraders. J. Med. Chem. 63, 11330–11361 (2020).
Dauvois, S., Danielian, P. S., White, R., Parker, M. G. & Antiestrogen, I. C. I. 164,384 reduces cellular estrogen receptor content by increasing its turnover. Proc. Natl Acad. Sci. USA 89, 4037–4041 (1992).
Wu, Y.-L. et al. Structural basis for an unexpected mode of SERM-mediated ER antagonism. Mol. Cell 18, 413–424 (2005).
Kerres, N. et al. Chemically induced degradation of the oncogenic transcription factor BCL6. Cell Rep. 20, 2860–2875 (2017). This paper describes the discovery of potent monomeric degraders of the TF BCL-6 from a medicinal chemistry campaign focused on optimizing BCL-6 PPI inhibitors.
Bellenie, B. R. et al. Achieving in vivo target depletion through the discovery and optimization of benzimidazolone BCL6 degraders. J. Med. Chem. 63, 4047–4068 (2020).
Słabicki, M. et al. Small-molecule-induced polymerization triggers degradation of BCL6. Nature 588, 164–168 (2020). This study defines a molecular glue mechanism of action for a potent BCL-6 monomeric degrader that involves polymerization of BCL-6 followed by degradation.
Faust, T. B. et al. Structural complementarity facilitates E7820-mediated degradation of RBM39 by DCAF15. Nat. Chem. Biol. 16, 7–14 (2020).
Powell, C. E. et al. Selective degradation of GSPT1 by cereblon modulators identified via a focused combinatorial library. ACS Chem. Biol. 15, 2722–2730 (2020).
Mayor-Ruiz, C. et al. Rational discovery of molecular glue degraders via scalable chemical profiling. Nat. Chem. Biol. 16, 1199–1207 (2020).
Sakamoto, K. M. et al. Protacs: chimeric molecules that target proteins to the Skp1–Cullin-F box complex for ubiquitination and degradation. Proc. Natl Acad. Sci. USA 98, 8554–8559 (2001).
Paiva, S.-L. & Crews, C. M. Targeted protein degradation: elements of PROTAC design. Curr. Opin. Chem. Biol. 50, 111–119 (2019).
Nalawansha, D. A. & Crews, C. M. PROTACs: an emerging therapeutic modality in precision medicine. Cell Chem. Biol. 27, 998–1014 (2020). This modern review succinctly highlights the advantages of PROTAC approaches and lays out a road map for the future development of this technology.
Bai, L. et al. A potent and selective small-molecule degrader of STAT3 achieves complete tumor regression in vivo. Cancer Cell 36, 498–511.e17 (2019). This paper showcases the advantages of degrading the STAT3 TF with PROTACs over the more traditional approach of disrupting homodimerization.
Gechijian, L. N. et al. Functional TRIM24 degrader via conjugation of ineffectual bromodomain and VHL ligands. Nat. Chem. Biol. 14, 405–412 (2018).
Bassi, Z. I. et al. Modulating PCAF/GCN5 immune cell function through a PROTAC approach. ACS Chem. Biol. 13, 2862–2867 (2018).
Cromm, P. M., Samarasinghe, K. T. G., Hines, J. & Crews, C. M. Addressing kinase-independent functions of Fak via PROTAC-mediated degradation. J. Am. Chem. Soc. 140, 17019–17026 (2018).
Degorce, S. L. et al. Discovery of proteolysis-targeting chimera molecules that selectively degrade the IRAK3 pseudokinase. J. Med. Chem. 63, 10460–10473 (2020).
Bondeson, D. P. et al. Catalytic in vivo protein knockdown by small-molecule PROTACs. Nat. Chem. Biol. 11, 611–617 (2015). This paper is the first to demonstrate that von Hippel–Lindau disease tumour suppressor (VHL) ligands can serve as E3-ligase recruiting modules for PROTACs and also describes the catalytic mechanism of action of VHL-based degraders.
Fisher, S. L. & Phillips, A. J. Targeted protein degradation and the enzymology of degraders. Curr. Opin. Chem. Biol. 44, 47–55 (2018).
Buhimschi, A. D. et al. Targeting the C481S ibrutinib-resistance mutation in Bruton’s tyrosine kinase using PROTAC-mediated degradation. Biochemistry 57, 3564–3575 (2018).
Burslem, G. M. et al. The advantages of targeted protein degradation over inhibition: an RTK case study. Cell Chem. Biol. 25, 67–77.e3 (2018).
Mares, A. et al. Extended pharmacodynamic responses observed upon PROTAC-mediated degradation of RIPK2. Commun. Biol. 3, 140 (2020).
Huang, H.-T. et al. A chemoproteomic approach to query the degradable kinome using a multi-kinase degrader. Cell Chem. Biol. 25, 88–99.e6 (2018).
Nowak, R. P. et al. Plasticity in binding confers selectivity in ligand-induced protein degradation. Nat. Chem. Biol. 14, 706–714 (2018).
Bondeson, D. P. et al. Lessons in PROTAC design from selective degradation with a promiscuous warhead. Cell Chem. Biol. 25, 78–87.e5 (2018).
Brand, M. et al. Homolog-selective degradation as a strategy to probe the function of CDK6 in AML. Cell Chem. Biol. 26, 300–306.e9 (2019).
Donovan, K. A. et al. Mapping the degradable kinome provides a resource for expedited degrader development. Cell 183, 1714–1731.e10 (2020). This study describes the characterization of a large library of kinase PROTACs using chemoproteomics and demonstrates that the most potent and selective PROTACs are not necessarily derived from the most potent and selective inhibitors.
Mullard, A. First targeted protein degrader hits the clinic. Nat. Rev. Drug Discov. 18, 237–239 (2019).
Lipinski, C. A., Lombardo, F., Dominy, B. W. & Feeney, P. J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 46, 3–26 (2001).
Li, Y. et al. Discovery of MD-224 as a first-in-class, highly potent, and efficacious proteolysis targeting chimera murine double minute 2 degrader capable of achieving complete and durable tumor regression. J. Med. Chem. 62, 448–466 (2019).
Winter, G. E. et al. Phthalimide conjugation as a strategy for in vivo target protein degradation. Science 348, 1376–1381 (2015). This study is the first to demonstrate that IMiDs, which are primarily ligands of the E3 ligase CRBN, can be used as E3-ligase recruiting modules for PROTACs.
Zengerle, M., Chan, K.-H. & Ciulli, A. Selective small molecule induced degradation of the BET bromodomain protein BRD4. ACS Chem. Biol. 10, 1770–1777 (2015).
Faivre, E. J. et al. Selective inhibition of the BD2 bromodomain of BET proteins in prostate cancer. Nature 578, 306–310 (2020).
Gilan, O. et al. Selective targeting of BD1 and BD2 of the BET proteins in cancer and immuno-inflammation. Science 368, 387–394 (2020).
Iniguez, A. B. et al. EWS/FLI confers tumor cell synthetic lethality to CDK12 inhibition in Ewing sarcoma. Cancer Cell 33, 202–216.e6 (2018).
Lasko, L. M. et al. Discovery of a selective catalytic p300/CBP inhibitor that targets lineage-specific tumours. Nature 550, 128–132 (2017). This paper describes the development of the first potent and selective inhibitor of the HAT domain of the related transcriptional co-activators CBP and p300.
Schick, S. et al. Acute BAF perturbation causes immediate changes in chromatin accessibility. Nat. Genet. 53, 269–278 (2021).
Osborne, J., Panova, S., Rapti, M., Urushima, T. & Jhoti, H. Fragments: where are we now? Biochem. Soc. Trans. 48, 271–280 (2020).
Li, Q. Application of fragment-based drug discovery to versatile targets. Front. Mol. Biosci. 7, 180 (2020).
Riback, J. A. et al. Innovative scattering analysis shows that hydrophobic disordered proteins are expanded in water. Science 358, 238–241 (2017).
Marsh, J. A. & Forman-Kay, J. D. Sequence determinants of compaction in intrinsically disordered proteins. Biophys. J. 98, 2383–2390 (2010).
Banks, A., Qin, S., Weiss, K. L., Stanley, C. B. & Zhou, H.-X. Intrinsically disordered protein exhibits both compaction and expansion under macromolecular crowding. Biophys. J. 114, 1067–1079 (2018).
Demarest, S. J. et al. Mutual synergistic folding in recruitment of CBP/p300 by p160 nuclear receptor coactivators. Nature 415, 549–553 (2002).
Kjaergaard, M., Teilum, K. & Poulsen, F. M. Conformational selection in the molten globule state of the nuclear coactivator binding domain of CBP. Proc. Natl Acad. Sci. USA 107, 12535–12540 (2010).
Naganathan, A. N. & Orozco, M. The native ensemble and folding of a protein molten-globule: functional consequence of downhill folding. J. Am. Chem. Soc. 133, 12154–12161 (2011).
Schneider, R. et al. Visualizing the molecular recognition trajectory of an intrinsically disordered protein using multinuclear relaxation dispersion NMR. J. Am. Chem. Soc. 137, 1220–1229 (2015).
Adamski, W. et al. A unified description of intrinsically disordered protein dynamics under physiological conditions using NMR spectroscopy. J. Am. Chem. Soc. 141, 17817–17829 (2019).
Milles, S., Salvi, N., Blackledge, M. & Jensen, M. R. Characterization of intrinsically disordered proteins and their dynamic complexes: from in vitro to cell-like environments. Prog. Nucl. Magn. Reson. Spectrosc. 109, 79–100 (2018).
Krishnan, N. et al. Targeting the disordered C terminus of PTP1B with an allosteric inhibitor. Nat. Chem. Biol. 10, 558–566 (2014).
Heller, G. T., Bonomi, M. & Vendruscolo, M. Structural ensemble modulation upon small-molecule binding to disordered proteins. J. Mol. Biol. 430, 2288–2292 (2018).
Heller, G. T. et al. Small-molecule sequestration of amyloid-β as a drug discovery strategy for Alzheimer’s disease. Sci. Adv. 6, eabb5924 (2020).
Flanagan, J. J. & Neklesa, T. K. Targeting nuclear receptors with PROTAC degraders. Mol. Cell. Endocrinol. 493, 110452 (2019).
Han, X. et al. Discovery of highly potent and efficient PROTAC degraders of androgen receptor (AR) by employing weak binding affinity VHL E3 ligase ligands. J. Med. Chem. 62, 11218–11231 (2019).
Hu, J. et al. Discovery of ERD-308 as a highly potent proteolysis targeting chimera (PROTAC) degrader of estrogen receptor (ER). J. Med. Chem. 62, 1420–1442 (2019).
Kargbo, R. B. PROTAC-mediated degradation of estrogen receptor in the treatment of cancer. ACS Med. Chem. Lett. 10, 1367–1369 (2019).
Silva, M. C. et al. Targeted degradation of aberrant tau in frontotemporal dementia patient-derived neuronal cell models. eLife 8, e45457 (2019).
Li, H. et al. Discovery of small-molecule inhibitors selectively targeting the DNA-binding domain of the human androgen receptor. J. Med. Chem. 57, 6458–6467 (2014).
Lee, G. T. et al. Effects of MTX-23, a novel PROTAC of androgen receptor splice variant-7 and androgen receptor, on CRPC resistant to second-line antiandrogen therapy. Mol. Cancer Ther. 20, 490–499 (2021).
Schapira, M., Calabrese, M. F., Bullock, A. N. & Crews, C. M. Targeted protein degradation: expanding the toolbox. Nat. Rev. Drug Discov. 18, 949–963 (2019).
Schneekloth, A. R., Pucheault, M., Tae, H. S. & Crews, C. M. Targeted intracellular protein degradation induced by a small molecule: en route to chemical proteomics. Bioorg. Med. Chem. Lett. 18, 5904–5908 (2008).
Itoh, Y., Ishikawa, M., Naito, M. & Hashimoto, Y. Protein knockdown using methyl bestatin−ligand hybrid molecules: design and synthesis of inducers of ubiquitination-mediated degradation of cellular retinoic acid-binding proteins. J. Am. Chem. Soc. 132, 5820–5826 (2010).
Zhang, X., Crowley, V. M., Wucherpfennig, T. G., Dix, M. M. & Cravatt, B. F. Electrophilic PROTACs that degrade nuclear proteins by engaging DCAF16. Nat. Chem. Biol. 15, 737–746 (2019).
Ward, C. C. et al. Covalent ligand screening uncovers a RNF4 E3 ligase recruiter for targeted protein degradation applications. ACS Chem. Biol. 14, 2430–2440 (2019).
Spradlin, J. N. et al. Harnessing the anti-cancer natural product nimbolide for targeted protein degradation. Nat. Chem. Biol. 15, 747–755 (2019).
Tong, B. et al. Bardoxolone conjugation enables targeted protein degradation of BRD4. Sci. Rep. 10, 15543 (2020).
Luo, M. et al. Chemoproteomics-enabled discovery of covalent RNF114-based degraders that mimic natural product function. Cell Chem. Biol. 28, 559–566.e15 (2021).
Molina, D. M. et al. Monitoring drug target engagement in cells and tissues using the cellular thermal shift assay. Science 341, 84–87 (2013).
Burslem, G. M., Bondeson, D. P. & Crews, C. M. Scaffold hopping enables direct access to more potent PROTACs with in vivo activity. Chem. Commun. 56, 6890–6892 (2020).
Schreiber, S. L. A chemical biology view of bioactive small molecules and a binder-based approach to connect biology to precision medicines. Isr. J. Chem. 59, 52–59 (2019).
Bradner, J. E. et al. A robust small-molecule microarray platform for screening cell lysates. Chem. Biol. 13, 493–504 (2006).
Bradner, J. E., McPherson, O. M. & Koehler, A. N. A method for the covalent capture and screening of diverse small molecules in a microarray format. Nat. Protoc. 1, 2344–2352 (2006).
Backus, K. M. et al. Proteome-wide covalent ligand discovery in native biological systems. Nature 534, 570–574 (2016).
Pop, M. S. et al. A small molecule that binds and inhibits the ETV1 transcription factor oncoprotein. Mol. Cancer Ther. 13, 1492–1502 (2014).
Yang, Z., Koehler, A. N. & Wang, L. A novel small molecule activator of nuclear receptor SHP inhibits HCC cell migration via suppressing Ccl2. Mol. Cancer Ther. 15, 2294–2301 (2016).
Roberts, A. M. et al. Chemoproteomic screening of covalent ligands reveals UBA5 as a novel pancreatic cancer target. ACS Chem. Biol. 12, 899–904 (2017).
Resnick, E. et al. Rapid covalent-probe discovery by electrophile-fragment screening. J. Am. Chem. Soc. 141, 8951–8968 (2019).
Ostrem, J. M., Peters, U., Sos, M. L., Wells, J. A. & Shokat, K. M. K-Ras(G12C) inhibitors allosterically control GTP affinity and effector interactions. Nature 503, 548–551 (2013).
Canon, J. et al. The clinical KRAS(G12C) inhibitor AMG 510 drives anti-tumour immunity. Nature 575, 217–223 (2019).
Hallin, J. et al. The KRASG12C inhibitor MRTX849 provides insight toward therapeutic susceptibility of KRAS-mutant cancers in mouse models and patients. Cancer Discov. 10, 54–71 (2020).
Boike, L. et al. Discovery of a functional covalent ligand targeting an intrinsically disordered cysteine within MYC. Cell Chem. Biol. 28, 4–13.e17 (2021). This paper describes the discovery of a covalent inhibitor of a disordered region of MYC, illustrating the power of covalent approaches for targeting challenging TFs.
Brenner, S. & Lerner, R. A. Encoded combinatorial chemistry. Proc. Natl Acad. Sci. USA 89, 5381–5383 (1992).
Goodnow, R. A., Dumelin, C. E. & Keefe, A. D. DNA-encoded chemistry: enabling the deeper sampling of chemical space. Nat. Rev. Drug Discov. 16, 131–147 (2017).
Neri, D. & Lerner, R. A. DNA-encoded chemical libraries: a selection system based on endowing organic compounds with amplifiable information. Annu. Rev. Biochem. 87, 479–502 (2018).
Gerry, C. J., Wawer, M. J., Clemons, P. A. & Schreiber, S. L. DNA barcoding a complete matrix of stereoisomeric small molecules. J. Am. Chem. Soc. 141, 10225–10235 (2019).
Gerry, C. J. & Schreiber, S. L. Recent achievements and current trajectories of diversity-oriented synthesis. Curr. Opin. Chem. Biol. 56, 1–9 (2020).
Bunnage, M. E., Gilbert, A. M., Jones, L. H. & Hett, E. C. Know your target, know your molecule. Nat. Chem. Biol. 11, 368–372 (2015).
Mo, H. & Henriksson, M. Identification of small molecules that induce apoptosis in a Myc-dependent manner and inhibit Myc-driven transformation. Proc. Natl Acad. Sci. USA 103, 6344–6349 (2006).
Wang, H. et al. Improved low molecular weight Myc-Max inhibitors. Mol. Cancer Ther. 6, 2399–2408 (2007).
Castell, A. et al. A selective high affinity MYC-binding compound inhibits MYC:MAX interaction and MYC-dependent tumor cell proliferation. Sci. Rep. 8, 10064 (2018).
Jafari, R. et al. The cellular thermal shift assay for evaluating drug target interactions in cells. Nat. Protoc. 9, 2100–2122 (2014).
Smith, E. & Collins, I. Photoaffinity labeling in target- and binding-site identification. Future Med. Chem. 7, 159–183 (2015).
Gao, J., Mfuh, A., Amako, Y. & Woo, C. M. Small molecule interactome mapping by photoaffinity labeling reveals binding site hotspots for the NSAIDs. J. Am. Chem. Soc. 140, 4259–4268 (2018).
Franken, H. et al. Thermal proteome profiling for unbiased identification of direct and indirect drug targets using multiplexed quantitative mass spectrometry. Nat. Protoc. 10, 1567–1593 (2015).
Iacobucci, C. et al. Carboxyl-photo-reactive MS-cleavable cross-linkers: unveiling a hidden aspect of diazirine-based reagents. Anal. Chem. 90, 2805–2809 (2018).
Weerapana, E. et al. Quantitative reactivity profiling predicts functional cysteines in proteomes. Nature 468, 790–795 (2010).
Wang, C., Weerapana, E., Blewett, M. M. & Cravatt, B. F. A chemoproteomic platform to quantitatively map targets of lipid-derived electrophiles. Nat. Methods 11, 79–85 (2014).
Nijman, S. M. B. Functional genomics to uncover drug mechanism of action. Nat. Chem. Biol. 11, 942–948 (2015).
Jost, M. et al. Combined CRISPRi/a-based chemical genetic screens reveal that rigosertib is a microtubule-destabilizing agent. Mol. Cell 68, 210–223.e6 (2017).
Neggers, J. E. et al. Target identification of small molecules using large-scale CRISPR–Cas mutagenesis scanning of essential genes. Nat. Commun. 9, 502 (2018).
Klein, I. A. et al. Partitioning of cancer therapeutics in nuclear condensates. Science 368, 1386–1392 (2020).
Siriwardena, S. U. et al. Phosphorylation-inducing chimeric small molecules. J. Am. Chem. Soc. 142, 14052–14057 (2020).
Erkizan, H. V. et al. A small molecule blocking oncogenic protein EWS-FLI1 interaction with RNA helicase A inhibits growth of Ewing’s sarcoma. Nat. Med. 15, 750–756 (2009).
Ponnusamy, S. et al. Novel selective agents for the degradation of androgen receptor variants to treat castration-resistant prostate cancer. Cancer Res. 77, 6282–6298 (2017).
Hwang, D.-J. et al. New generation of selective androgen receptor degraders: our initial design, synthesis, and biological evaluation of new compounds with enzalutamide-resistant prostate cancer activity. J. Med. Chem. 62, 491–511 (2019).
Kent, L. N. & Leone, G. The broken cycle: E2F dysfunction in cancer. Nat. Rev. Cancer 19, 326–338 (2019).
Sanda, T. et al. Core transcriptional regulatory circuit controlled by the TAL1 complex in human T cell acute lymphoblastic leukemia. Cancer Cell 22, 209–221 (2012).
Gryder, B. E. et al. Miswired enhancer logic drives a cancer of the muscle lineage. iScience 23, 101103 (2020).
Sikorski, K., Czerwoniec, A., Bujnicki, J. M., Wesoly, J. & Bluyssen, H. A. R. STAT1 as a novel therapeutical target in pro-atherogenic signal integration of IFNγ, TLR4 and IL-6 in vascular disease. Cytokine Growth Factor Rev. 22, 211–219 (2011).
Acknowledgements
The authors acknowledge funding received from the National Institutes of Health (NIH) (U54CA231630-01A1) and the Emerson Collective.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
A.N.K. is a founder of Kronos Bio and is both a shareholder and a member of the Scientific Advisory Board.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Non-specific TF binding sites
-
Sequences of DNA that do not contain the consensus sequence for a transcription factor (TF) DNA-binding domain (DBD). Most DBDs have low affinity for non-specific sites, but because of the exceptionally high ratio of non-specific to specific sites, TFs often spend significant time at non-specific sites.
- Specific TF binding sites
-
Sequences of DNA that contain the consensus sequence for a transcription factor (TF) DNA-binding domain.
- Transcriptional condensates
-
Liquid–liquid phase-separated droplets containing transcription factors, co-activators, RNA polymerase II (Pol II) and other transcriptional machinery.
- Pre-initiation complex
-
A large complex comprising general transcription factors, Mediator and other proteins that position and activate RNA polymerase II (Pol II) at the transcription start site.
- Cooperativity
-
In transcription, a phenomenon where binding of one transcription factor and/or co-regulator at a regulatory element enhances the binding of other factors, and vice versa.
- Core regulatory TFs
-
(Also known as master TFs). Self-regulated transcription factors (TFs) that control cell identity and fate.
- Chromatin readers
-
Proteins, such as bromodomains, that bind to post-translationally modified histones.
- Ubiquitin–proteasome system
-
A system of intracellular protein degradation that is mediated by transfer of ubiquitin to target proteins by ubiquitin E3 ligases to mark them for degradation by the proteasome.
- Molecular glues
-
Small molecules that directly mediate a non-native protein–protein interaction.
- Histone deacetylases
-
(HDACs). Enzymes that remove acetyl groups from acetylated Lys residues in histones. Generally associated with closed chromatin conformation and transcriptional repression.
- Histone acetyltransferases
-
(HATs). Enzymes that transfer acetyl groups to the ε-amino group of Lys residues in histones. Generally associated with open chromatin conformation and increased transcription.
- Chromatin remodellers
-
Protein complexes with a common ATPase domain that use ATP hydrolysis to move, reposition or eject nucleosomes.
- Intrinsically disordered proteins
-
(IDPs; also known as intrinsically disordered regions (IDRs)). Proteins or regions of proteins that do not adopt a well-defined structure. Often characterized as ‘ensembles’ of many unrelated protein conformations, although many IDPs/IDRs can transiently form more defined structures.
- NMR spectroscopy
-
A structural technique that utilizes the quantum-mechanical properties of nuclear spins in a magnetic field. Used in structural biology to determine protein structure, as well as to characterize conformational dynamics across a wide range of timescales (general range of picoseconds to hours/days).
- Molecular dynamics
-
A computational technique that is used to characterize the structure and conformational dynamics of proteins by simulating the interactions between all the atoms of a protein and its surrounding solvent over time.
- Occupancy-based modulators
-
Modulators of protein function in drug discovery where the overall change in protein activity from drug treatment is determined by the concentration of the drug in the cell and its affinity for its target. For complete inhibition of activity by an inhibitor, the drug must reach concentrations several times above its dissociation constant (Kd).
- ARV7
-
A splice variant of the androgen receptor that lacks the ligand-binding domain.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Henley, M.J., Koehler, A.N. Advances in targeting ‘undruggable’ transcription factors with small molecules. Nat Rev Drug Discov 20, 669–688 (2021). https://doi.org/10.1038/s41573-021-00199-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41573-021-00199-0
This article is cited by
-
Identification of FDA-approved drugs that induce heart regeneration in mammals
Nature Cardiovascular Research (2024)
-
CRISPR-Cas9 knockout screening identifies KIAA1429 as an essential gene in Ewing sarcoma
Journal of Experimental & Clinical Cancer Research (2023)
-
Novel cancer treatment paradigm targeting hypoxia-induced factor in conjunction with current therapies to overcome resistance
Journal of Experimental & Clinical Cancer Research (2023)
-
The role and regulation of Maf proteins in cancer
Biomarker Research (2023)
-
Recent advances in targeting the “undruggable” proteins: from drug discovery to clinical trials
Signal Transduction and Targeted Therapy (2023)