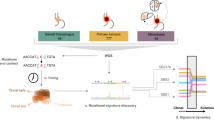
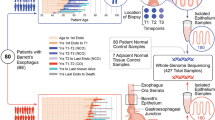
Abstract
Cancer cells are shaped through an evolutionary process of DNA mutation, cell selection and population expansion. Early steps in this process are driven by a set of mutated driver genes and structural alterations to the genome through copy number gains or losses. Oesophageal adenocarcinoma (EAC) and the pre-invasive tissue, Barrett’s oesophagus (BE), provide an ideal example in which to observe and study this evolution. BE displays early genomic instability, specifically in copy number changes that may later be observed in EAC. Furthermore, these early changes result in patterns of progression (that is, ‘born bad’, gradual or catastrophic) that may help to describe the evolution of EAC. As only a small proportion of patients with BE will go on to develop cancer, a better understanding of these patterns and the resulting genomic changes should improve early detection in EAC and may provide clues for the evolution of cancer more broadly.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Gerstung, M. et al. The evolutionary history of 2,658 cancers. Nature 578, 122–128 (2020). This work, coming out of the largest pan-cancer analysis to date, establishes the concept of early and late mutations in the evolution of tumours, suggesting that early gene mutations might be detectable and offer the potential of earlier treatment.
Li, Y. et al. Patterns of somatic structural variation in human cancer genomes. Nature 578, 112–121 (2020).
Zack, T. I. et al. Pan-cancer patterns of somatic copy number alteration. Nat. Genet. 45, 1134–1140 (2013).
Alexandrov, L. B., Nik-Zainal, S., Wedge, D. C., Campbell, P. J. & Stratton, M. R. Deciphering signatures of mutational processes operative in human cancer. Cell Rep. 3, 246–259 (2013).
Ross-Innes, C. S. et al. Whole-genome sequencing provides new insights into the clonal architecture of Barrett’s esophagus and esophageal adenocarcinoma. Nat. Genet. 47, 1038–1046 (2015).
ICGC/TCGA. Pan-cancer analysis of whole genomes. Nature 578, 82–93 (2020).
Alexandrov, L. B., Kim, J., Haradhvala, N. J. & Huang, M. N. The repertoire of mutational signatures in human cancer. Nature 578, 94–101 (2020).
Martincorena, I. et al. High burden and pervasive positive selection of somatic mutations in normal human skin. Science 348, 880–886 (2015).
Hoang, M. L. et al. Genome-wide quantification of rare somatic mutations in normal human tissues using massively parallel sequencing. Proc. Natl Acad. Sci. USA 113, 9846–9851 (2016).
Brunner, S. F. et al. Somatic mutations and clonal dynamics in healthy and cirrhotic human liver. Nature 574, 538–542 (2019).
Lee-Six, H. et al. Population dynamics of normal human blood inferred from somatic mutations. Nature 561, 473–478 (2018).
Yizhak, K. et al. RNA sequence analysis reveals macroscopic somatic clonal expansion across normal tissues. Science 364, eaaw0726 (2019).
Stratton, M. R., Campbell, P. J. & Futreal, P. A. The cancer genome. Nature 458, 719–724 (2009).
Martincorena, I. & Campbell, P. J. Somatic mutation in cancer and normal cells. Science 349, 961–968 (2016).
Jakubek, Y. A. et al. Large-scale analysis of acquired chromosomal alterations in non-tumor samples from patients with cancer. Nat. Biotechnol. 38, 90–96 (2020).
Reid, B. J., Li, X., Galipeau, P. C. & Vaughan, T. L. Barrett’s oesophagus and oesophageal adenocarcinoma: time for a new synthesis. Nat. Rev. Cancer 10, 87–101 (2010).
Spechler, S. J. Carcinogenesis at the gastroesophageal junction: free radicals at the frontier. Gastroenterology 122, 1518–1520 (2002).
Ferlay, J. et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 136, E359–E386 (2015).
Thrift, A. P. Global burden and epidemiology of Barrett oesophagus and oesophageal cancer. Nat. Rev. Gastroenterol. Hepatol. 18, 432–443 (2021).
Hvid-Jensen, F. et al. Incidence of adenocarcinoma among patients with Barrett’s esophagus. N. Engl. J. Med. 365, 1375–1383 (2011). This work is one of the largest population-based studies to show that the risk of progression from non-dysplastic BE to EAC is very low (≤0.3% per year).
Smyth, E. C. et al. Oesophageal cancer. Nat. Rev. Dis. Prim. 3, 17048 (2017).
Wani, S., Rubenstein, J. H., Vieth, M. & Bergman, J. Diagnosis and management of low-grade dysplasia in Barrett’s esophagus: expert review from the clinical practice updates committee of the American Gastroenterological Association. Gastroenterology 151, 822–835 (2016).
Killcoyne, S. et al. Identification of prognostic phenotypes of esophageal adenocarcinoma in two independent cohorts. Gastroenterology 155, 1720–1728 (2018).
Bhat, S. K. et al. Oesophageal adenocarcinoma and prior diagnosis of Barrett’s oesophagus: a population-based study. Gut 64, 20–25 (2015).
The Cancer Genome Atlas Research Network. Integrated genomic characterization of oesophageal carcinoma. Nature 541, 169–175 (2017).
Frankell, A. M. et al. The landscape of selection in 551 esophageal adenocarcinomas defines genomic biomarkers for the clinic. Nat. Genet. 51, 506–516 (2019).
Secrier, M. et al. Mutational signatures in esophageal adenocarcinoma define etiologically distinct subgroups with therapeutic relevance. Nat. Genet. 48, 1131–1141 (2016).
Nik-Zainal, S. et al. Mutational processes molding the genomes of 21 breast cancers. Cell 149, 979–993 (2012).
Maley, C. C. et al. The combination of genetic instability and clonal expansion predicts progression to esophageal adenocarcinoma. Cancer Res. 64, 7629–7633 (2004).
Martinez, P. et al. Dynamic clonal equilibrium and predetermined cancer risk in Barrett’s oesophagus. Nat. Commun. 7, 12158 (2016).
Killcoyne, S. et al. Genomic copy number predicts esophageal cancer years before transformation. Nat. Med. 26, 1726–1732 (2020).
Alexandrov, L. B. et al. Signatures of mutational processes in human cancer. Nature 500, 415–421 (2013). This paper together with Alexandrov et al. (Cell Reports, 2013 and Nature, 2020) establishes the concept of mutational signatures, helping to study mutational processes active within the somatic genome.
Newell, F. et al. Complex structural rearrangements are present in high-grade dysplastic Barrett’s oesophagus samples. BMC Med. Genomics 12, 31 (2019).
Maley, C. C. et al. Genetic clonal diversity predicts progression to esophageal adenocarcinoma. Nat. Genet. 38, 468–473 (2006). Together with Maley et al. (‘The combination …’, Cancer Research, 2004), this paper provides evidence that early genetic instability, rather than individual biomarkers, can be used to predict the risk of progression in a single patient.
Dulak, A. M. et al. Exome and whole-genome sequencing of esophageal adenocarcinoma identifies recurrent driver events and mutational complexity. Nat. Genet. 45, 478–486 (2013). This paper from TCGA alongside Secrier et al. (2016) and Frankell et al. (2019), provides a comprehensive genomic characterization of EAC from the largest patient cohorts to date.
Nones, K. et al. Genomic catastrophes frequently arise in esophageal adenocarcinoma and drive tumorigenesis. Nat. Commun. 5, 5224 (2014).
Weaver, J. M. J. et al. Ordering of mutations in preinvasive disease stages of esophageal carcinogenesis. Nat. Genet. 46, 837–843 (2014).
Stachler, M. D. et al. Paired exome analysis of Barrett’s esophagus and adenocarcinoma. Nat. Genet. 47, 1047–1055 (2015).
Christensen, S. et al. 5-Fluorouracil treatment induces characteristic T > G mutations in human cancer. Nat. Commun. 10, 1–11 (2019).
Tomkova, M., Tomek, J., Kriaucionis, S. & Schuster-Böckler, B. Mutational signature distribution varies with DNA replication timing and strand asymmetry. Genome Biol. 19, 129 (2018).
Pich, O. et al. Somatic and germline mutation periodicity follow the orientation of the DNA minor groove around nucleosomes. Cell 175, 1074–1087.e18 (2018).
Gonzalez-Perez, A., Sabarinathan, R. & Lopez-Bigas, N. Local determinants of the mutational landscape of the human genome. Cell 177, 101–114 (2019).
Bass, A. J. et al. Comprehensive molecular characterization of gastric adenocarcinoma. Nature 513, 202–209 (2014).
Martincorena, I. et al. Universal patterns of selection in cancer and somatic tissues. Cell 171, 1029–1041.e21 (2017).
Stachler, M. D. et al. Detection of mutations in Barrett’s esophagus before progression to high-grade dysplasia or adenocarcinoma. Gastroenterology 155, 156–167 (2018).
Ross-Innes, C. S. et al. Evaluation of a minimally invasive cell sampling device coupled with assessment of trefoil factor 3 expression for diagnosing barrett’s esophagus: a multi-center case–control study. PLoS Med. 12, e1001780 (2015).
Sottoriva, A. et al. A big bang model of human colorectal tumor growth. Nat. Genet. 47, 209–216 (2015).
Martinez, P. et al. Evolution of Barrett’s esophagus through space and time at single-crypt and whole-biopsy levels. Nat. Commun. 9, 794 (2018).
Liu, Y. et al. Comparative molecular analysis of gastrointestinal adenocarcinomas. Cancer Cell 33, 721–735.e8 (2018).
Noorani, A. et al. Genomic evidence supports a clonal diaspora model for metastases of esophageal adenocarcinoma. Nat. Genet. 52, 74–83 (2020).
Saito, T. et al. A temporal shift of the evolutionary principle shaping intratumor heterogeneity in colorectal cancer. Nat. Commun. 9, 1–11 (2018).
Wu, H. et al. Evolution and heterogeneity of non-hereditary colorectal cancer revealed by single-cell exome sequencing. Oncogene 36, 2857–2867 (2017).
Dulak, A. M. et al. Gastrointestinal adenocarcinomas of the esophagus, stomach, and colon exhibit distinct patterns of genome instability and oncogenesis. Cancer Res. 72, 4383–4394 (2012).
Hadi, K. et al. Distinct classes of complex structural variation uncovered across thousands of cancer genome graphs. Cell 183, 197–210.e32 (2020).
Jakubek, Y. et al. Genomic landscape established by allelic imbalance in the cancerization field of a normal appearing airway. Cancer Res. 76, 3676–3683 (2016).
Conconi, D. et al. Unexpected frequency of genomic alterations in histologically normal colonic tissue from colon cancer patients. Tumor Biol. 37, 13831–13842 (2016).
Burrell, R. A., McGranahan, N., Bartek, J. & Swanton, C. The causes and consequences of genetic heterogeneity in cancer evolution. Nature 501, 338–345 (2013).
Galipeau, P. C. et al. 17p (p53) allelic losses, 4N (G2/tetraploid) populations, and progression to aneuploidy in Barrett’s esophagus. Proc. Natl Acad. Sci. USA 93, 7081–7084 (1996).
Barrett, M., Galipeau, P., Sanchez, C., Emond, M. & Reid, B. Determination of the frequency of loss of heterozygosity in esophageal adenocarcinoma by cell sorting, whole genome amplification and microsatellite polymorphisms. Oncogene 12, 1873–1878 (1996).
Barrett, M. T. et al. Allelic loss of 9p21 and mutation of the CDKN2/p16 gene develop as early lesions during neoplastic progression in Barrett’s esophagus. Oncogene 13, 1867–1873 (1996).
Galipeau, P. C., Prevo, L. J., Sanchez, C. A., Longton, G. M. & Reid, B. J. Clonal expansion and loss of heterozygosity at chromosomes 9p and 17p in premalignant esophageal (Barrett’s) tissue. JNCI 91, 2087–2095 (1999).
Maley, C. C. et al. Selectively advantageous mutations and hitchhikers in neoplasms: p16 lesions are selected in Barrett’s esophagus. Cancer Res. 64, 3414–3427 (2004).
Li, X. et al. Temporal and spatial evolution of somatic chromosomal alterations: a case–cohort study of Barrett’s esophagus. Cancer Prev. Res. 7, 114–127 (2014).
Levine, D. S., Reid, B. J., Haggitt, R. C., Rubin, C. E. & Rabinovitch, P. S. Correlation of ultrastructural aberrations with dysplasia and flow cytometric abnormalities in Barrett’s epithelium. Gastroenterology 96, 355–367 (1989).
Reid, B. et al. Flow-cytometric and histological progression to malignancy in Barrett’s esophagus: prospective endoscopic surveillance of a cohort. Gastroenterology 102, 1212–1219 (1992).
Reid, B. J. et al. Predictors of progression in Barrett’s esophagus II: baseline 17p (p53) loss of heterozygosity identifies a patient subset at increased risk for neoplastic progression. Am. J. Gastroenterol. 96, 2839–2848 (2001). Together with Galipeau et al. (1996), Galipeau et al. (1999) and Reid et al. (2001), this paper provides evidence that early copy number changes occur in BE and confer increased risk of cancer to patients with BE.
Rabinovitch, P. S., Longton, G., Blount, P. L., Levine, D. S. & Reid, B. J. Predictors of progression in Barrett’s esophagus III: baseline flow cytometric variables. Am. J. Gastroenterol. 96, 3071–3083 (2001).
Greaves, M. & Maley, C. C. Clonal evolution in cancer. Nature 481, 306–313 (2012).
Curtius, K. et al. A molecular clock infers heterogeneous tissue age among patients with Barrett’s esophagus. PLoS Comput. Biol. 12, e1004919 (2016).
Stephens, P. J. et al. Massive genomic rearrangement acquired in a single catastrophic event during cancer development. Cell 144, 27–40 (2011).
Gao, R. et al. Punctuated copy number evolution and clonal stasis in triple-negative breast cancer. Nat. Genet. 48, 1119–1130 (2016).
Bonnington, S. N. & Rutter, M. D. Surveillance of colonic polyps: are we getting it right? World J. Gastroenterol. 22, 1925–1934 (2016).
Cheng, Y.-W. et al. CpG island methylator phenotype associates with low-degree chromosomal abnormalities in colorectal cancer. Clin. Cancer Res. 14, 6005–6013 (2008).
The Cancer Genome Atlas Network. Comprehensive molecular characterization of human colon and rectal cancer. Nature 487, 330–337 (2012).
Baca, S. C. et al. Punctuated evolution of prostate cancer genomes. Cell 153, 666–677 (2013).
Ly, P. & Cleveland, D. W. Rebuilding chromosomes after catastrophe: emerging mechanisms of chromothripsis. Trends Cell Biol. 27, 917–930 (2017).
Killcoyne, S. & Fitzgerald, R. C. Practical early cancer detection: distinguishing stable from unstable genomes in pre-cancerous tissues. Br. J. Cancer 124, 683–685 (2020).
Shaheen, N. J., Falk, G. W., Iyer, P. G. & Gerson, L. B. ACG clinical guideline: diagnosis and management of Barrett’s esophagus. Am. J. Gastroenterol. 111, 30–50 (2016).
Fitzgerald, R. C. et al. British Society of Gastroenterology guidelines on the diagnosis and management of Barrett’s oesophagus. Gut 63, 7–42 (2014).
Bhat, S. et al. Risk of malignant progression in Barrett’s Esophagus patients: results from a large population-based study. J. Natl. Cancer Inst. 103, 1049–1057 (2011).
Shaheen, N. J. et al. Radiofrequency ablation in Barrett’s esophagus with dysplasia. N. Engl. J. Med. 360, 2277–2288 (2009).
Phoa, K. N. et al. Radiofrequency ablation vs endoscopic surveillance for patients with Barrett esophagus and low-grade dysplasia: a randomized clinical trial. JAMA 311, 1209–1217 (2014).
Fitzgerald, R. C. et al. Cytosponge-trefoil factor 3 versus usual care to identify Barrett’s oesophagus in a primary care setting: a multicentre, pragmatic, randomised controlled trial. Lancet 396, 333–344 (2020).
Davidson, M. et al. Detecting and tracking circulating tumour DNA copy number profiles during first line chemotherapy in oesophagogastric adenocarcinoma. Cancers 11, 736 (2019).
Babayan, A. & Pantel, K. Advances in liquid biopsy approaches for early detection and monitoring of cancer. Genome Med. 10, 21 (2018).
van der Wel, M. J. et al. Improved diagnostic stratification of digitised Barrett’s oesophagus biopsies by p53 immunohistochemical staining. Histopathology 72, 1015–1023 (2018).
Hamelin, R. et al. TP53 gene mutations and p53 protein immunoreactivity in malignant and premalignant Barrett’s esophagus. Gastroenterology 107, 1012–1018 (1994).
Bang, Y. J. et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 376, 687–697 (2010).
Davelaar, A. L. et al. Aberrant TP53 detected by combining immunohistochemistry and DNA-FISH improves Barrett’s esophagus progression prediction: a prospective follow-up study. Genes Chromosom. Cancer 54, 82–90 (2015).
Reid, B. J., Levine, D. S., Longton, G., Blount, P. L. & Rabinovitch, P. S. Predictors of progression to cancer in Barrett’s esophagus: baseline histology and flow cytometry identify low- and high-risk patient subsets. Am. J. Gastroenterol. 95, 1669–1676 (2000).
Hadjinicolaou, A. V. et al. Aneuploidy in targeted endoscopic biopsies outperforms other tissue biomarkers in the prediction of histologic progression of Barrett’s oesophagus: a multi-centre prospective cohort study. Ebiomedicine 56, 102765 (2020).
Li, X. et al. Assessment of esophageal adenocarcinoma risk using somatic chromosome alterations in longitudinal samples in Barrett’s esophagus. Cancer Prev. Res. 8, 845–856 (2015).
Douville, C. et al. Massively parallel sequencing of esophageal brushings enables an aneuploidy-based classification of patients with Barrett’s esophagus. Gastroenterology 160, 2043–2054 (2021).
Vaughan, T. L. & Fitzgerald, R. C. Precision prevention of oesophageal adenocarcinoma. Nat. Rev. Gastroenterol. Hepatol. 12, 243–248 (2015).
Parasa, S. et al. Development and validation of a model to determine risk of progression of Barrett’s esophagus to neoplasia. Gastroenterology 154, 1282–1289.e2 (2018).
Hardikar, S. et al. The role of tobacco, alcohol, and obesity in neoplastic progression to esophageal adenocarcinoma: a prospective study of Barrett’s esophagus. PLoS ONE 8, e52192 (2013).
Zagari, R. M. et al. Gastro-oesophageal reflux symptoms, oesophagitis and Barrett’s oesophagus in the general population: the Loiano-Monghidoro study. Gut 57, 1354–1359 (2008).
Ronkainen, J. et al. Prevalence of Barrett’s esophagus in the general population: an endoscopic study. Gastroenterology 129, 1825–1831 (2005).
Hamade, N. et al. Lower annual rate of progression of short-segment vs long-segment Barrett’s esophagus to esophageal adenocarcinoma. Clin. Gastroenterol. Hepatol. 17, 864–868 (2019).
Papaemmanuil, E. et al. Genomic classification and prognosis in acute myeloid leukemia. N. Engl. J. Med. 374, 2209–2221 (2016).
Gerstung, M. et al. Precision oncology for acute myeloid leukemia using a knowledge bank approach. Nat. Genet. 49, 332–340 (2017).
Cook, M. B. et al. Cigarette smoking increases risk of Barrett’s esophagus: an analysis of the Barrett’s and esophageal adenocarcinoma consortium. Gastroenterology 142, 744–753 (2012).
Vaughan, T. L., Onstad, L. & Dai, J. Y. Interactive decision support for esophageal adenocarcinoma screening and surveillance. BMC Gastroenterol. 19, 109 (2019).
Keswani, R. N., Noffsinger, A., Waxman, I. & Bissonnette, M. Clinical use of p53 in Barrett’s esophagus. Cancer Epidemiol. Biomarkers Prev. 15, 1243–1249 (2006).
Jin, Z. et al. A multicenter, double-blinded validation study of methylation biomarkers for progression prediction in Barrett’s esophagus. Cancer Res. 69, 4112–4115 (2009).
Sato, F. et al. Three-tiered risk stratification model to predict progression in Barrett’s esophagus using epigenetic and clinical features. PLoS ONE 3, e1890 (2008).
Souza, R. F. Reflux esophagitis and its role in the pathogenesis of Barrett’s metaplasia. J. Gastroenterol. 52, 767–776 (2017).
Wang, D. H. The esophageal squamous epithelial cell — still a reasonable candidate for the Barrett’s esophagus cell of origin? Cell. Mol. Gastroenterol. Hepatol. 4, 157–160 (2017).
Kong, J., Crissey, M. A., Funakoshi, S., Kreindler, J. L. & Lynch, J. P. Ectopic Cdx2 expression in murine esophagus models an intermediate stage in the emergence of Barrett’s esophagus. PLoS ONE 6, 18280 (2011).
Clemons, N. J. et al. Sox9 drives columnar differentiation of esophageal squamous epithelium: a possible role in the pathogenesis of Barrett’s esophagus. Am. J. Physiol. Gastrointest. Liver Physiol. 303, 1335–1346 (2012).
Owen, R. P. et al. Single cell RNA-seq reveals profound transcriptional similarity between Barrett’s oesophagus and oesophageal submucosal glands. Nat. Commun. 9, 4261 (2018).
Leedham, S. J. et al. Individual crypt genetic heterogeneity and the origin of metaplastic glandular epithelium in human Barrett’s oesophagus. Gut 57, 1041–1048 (2008).
Jiang, M. et al. Transitional basal cells at the squamous-columnar junction generate Barrett’s oesophagus. Nature 550, 529–533 (2017).
Wang, X. et al. Residual embryonic cells as precursors of a Barrett’s-like metaplasia. Cell 145, 1023–1035 (2011).
McQuaid, K. R., Laine, L., Fennerty, M. B., Souza, R. & Spechler, S. J. Systematic review: The role of bile acids in the pathogenesis of gastro-oesophageal reflux disease and related neoplasia. Aliment. Pharmacol. Ther. 34, 146–165 (2011).
Gokon, Y. et al. Immune microenvironment in Barrett’s esophagus adjacent to esophageal adenocarcinoma: possible influence of adjacent mucosa on cancer development and progression. Virchows Arch. 477, 825–834 (2020).
Fitzgerald, R. C. et al. Diversity in the oesophageal phenotypic response to gastro-oesophageal reflux: Immunological determinants. Gut 50, 451–459 (2002).
Kavanagh, M. E. et al. Impact of the inflammatory microenvironment on T-cell phenotype in the progression from reflux oesophagitis to Barrett oesophagus and oesophageal adenocarcinoma. Cancer Lett. 370, 117–124 (2016).
Lagisetty, K. H. et al. Immune determinants of Barrett’s progression to esophageal adenocarcinoma. JCI Insight 6, e143888 (2021). This paper provides an overview of the changes within the immune environment between BE and EAC.
Wagener-Ryczek, S. et al. Immune profile and immunosurveillance in treatment-naive and neoadjuvantly treated esophageal adenocarcinoma. Cancer Immunol. Immunother. 69, 523–533 (2020).
Galipeau, P. C. et al. NSAIDs modulate CDKN2A, TP53, and DNA content risk for progression to esophageal adenocarcinoma. PLoS Med. 4, e67 (2007).
Galipeau, P. C. et al. NSAID use and somatic exomic mutations in Barrett’s esophagus. Genome Med. 10, 17 (2018).
Liao, L. M. et al. Nonsteroidal anti-inflammatory drug use reduces risk of adenocarcinomas of the esophagus and esophagogastric junction in a pooled analysis. Gastroenterology 142, 442–452.e5; quiz e22–e23 (2012).
Jankowski, J. A. Z. et al. Esomeprazole and aspirin in Barrett’s oesophagus (AspECT): a randomised factorial trial. Lancet 392, 400–408 (2018). This paper presents AspECT, the largest clinical trial to date investigating the real effect of aspirin and proton-pump inhibitors to delay or inhibit EAC in patients with BE.
Acknowledgements
The laboratory of R.C.F. is funded by a Programme Grant from the Medical Research Council (MRC) (RG84369), and Cancer Research UK provided funding for the Oesophageal Cancer Clinical and Molecular Stratification (OCCAMS)/International Cancer Genome Consortium (ICGC) oesophageal cancer programme (RG66287).
Author information
Authors and Affiliations
Contributions
Both authors contributed equally to this article.
Corresponding author
Ethics declarations
Competing interests
R.C.F. is named on patents for Cytosponge and related assays that have been licensed by the Medical Research Council (MRC) to Covidien GI Solutions (now Medtronic). R.C.F is a co-founder and shareholder of Cyted Ltd, an Early Detection company. S.K. declares no competing interests.
Additional information
Peer review information
Nature Reviews Cancer thanks M. Stachler, T. Wang and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Acute myeloid leukaemia multistage predictions: https://cancer.sanger.ac.uk/aml-multistage/
GitHub — gerstung-lab/BarrettsProgressionRisk: https://github.com/gerstung-lab/BarrettsProgressionRisk
Interactive and Contextual RISk Calculator (IC-RISC): https://ic-risc.fredhutch.org/
Glossary
- Kataegis
-
Localized hypermutation often several hundred base pairs in length.
- Mutational signature
-
A combination of mutations (specifically single base-pair substitutions) that generates a specific pattern, or signature, relating to specific mutational processes.
- Minor-in groove
-
DNA facing in to the histone core (minor-in).
- Chromothripsis
-
Hundreds of clustered breaks occurring in a single catastrophic event affecting a limited number of chromosomes.
- Breakage–fusion–bridge processes
-
Mechanisms of genomic instability initiated by telomeric end fusions following double-stranded breaks, which can result in repetitive cycles of fusions and breaks.
- Extrachromosomal DNA
-
(Often extrachromosomal circular DNA). DNA found separate to the chromosomes and often contributing to higher copy numbers and altered gene expression in cancer.
- Chromoplexy
-
Chains of rearrangements that result from the repair of double-stranded breakages.
- Cytosponge
-
A non-endoscopic device for sampling cells within the oesophagus consisting of a pill with a sponge on a string that can be swallowed by the patient.
- AUC ROC
-
(Area under the receiver operating characteristic curve). A performance metric for classification at various thresholds by plotting the true positive rate against the false positive rate.
- Elastic-net regression model
-
A regularized regression method that combines the penalties of LASSO and ridge methods.
- Long-segment BE
-
Barrett’s oesophagus (BE) replaces normal squamous epithelium along measurable lengths of the oesophagus from <1 cm to ≥3 cm extending from the junction of the stomach and the oesophagus.
- Hiatal hernia
-
The upper part of the stomach bulges through the opening of the diaphragm (hiatus) into the oesophagus.
Rights and permissions
About this article
Cite this article
Killcoyne, S., Fitzgerald, R.C. Evolution and progression of Barrett’s oesophagus to oesophageal cancer. Nat Rev Cancer 21, 731–741 (2021). https://doi.org/10.1038/s41568-021-00400-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41568-021-00400-x
This article is cited by
-
Stage II oesophageal carcinoma: peril in disguise associated with cellular reprogramming and oncogenesis regulated by pseudogenes
BMC Genomics (2024)
-
Unveiling the mechanisms and challenges of cancer drug resistance
Cell Communication and Signaling (2024)
-
Comparative immunological landscape between pre- and early-stage LUAD manifested as ground-glass nodules revealed by scRNA and scTCR integrated analysis
Cell Communication and Signaling (2023)