Abstract
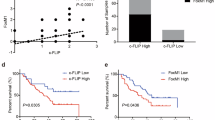
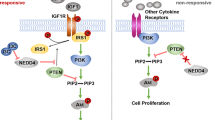
IFIT1 and IFIT3 are abundant products of interferon-stimulating genes. While the importance of IFIT1 and IFIT3 in the prognosis of cancer has been reported, the molecular basis of IFIT1 and IFIT3 in cancer progression remains unexplored. In the present study, we investigated the modes of action and the clinical significance of IFIT1 and IFIT3 in oral squamous cell carcinoma (OSCC). Ectopic expression of IFIT1 or IFIT3 induced OSCC cell invasion by promoting the epithelial-mesenchymal transition, whereas IFIT1 or IFIT3 knockdown exhibited opposite effects. Overexpression of IFIT1 or IFIT3 promoted tumor growth, regional and distant metastasis in xenograft and orthotopic nude mice models. Most importantly, IFIT1 or IFIT3 overexpression increased the levels of p-EGFRY1068 and p-AKTS473 in OSCC cells and also enhanced tumor inhibitory effect of gefitinib. By immunoprecipitation and LC-MS/MS analysis, we found that IFIT1 and IFIT3 interacted with ANXA2 that enhanced p-EGFRY1068 endosomal recycling. Depletion of ANXA2 using siRNA therefore abolished p-EGFRY1068 and p-AKTS473 expression in IFIT1- or IFIT3-overexpressed cells. Furthermore, a significant positive association of increased IFIT1 and IFIT3 expression with advanced T-stage, lymph node metastasis, perineural invasion, lymphovascular invasion, extranodal extension, and poor overall survival rate was confirmed in OSCC patients. We also found a statistically positive correlation of p-EGFRY1068 expression with IFIT1 and IFIT3 in OSCC tumors and poor clinical outcome in patients. Collectively, we demonstrated a novel role of IFIT1 and IFIT3 in driving OSCC progression and metastasis by interacting with ANXA2 and hence enhancing p-EGFR recycling and its downstream signaling.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Borden EC, Sen GC, Uze G, Silverman RH, Ransohoff RM, Foster GR, et al. Interferons at age 50: past, current and future impact on biomedicine. Nat Rev Drug Discov. 2007;6:975–90.
Parker BS, Rautela J, Hertzog PJ. Antitumour actions of interferons: implications for cancer therapy. Nat Rev Cancer. 2016;16:131–44.
Yang JL, Qu XJ, Hayes VM, Brenner PC, Russell PJ, Goldstein D. Erlotinib (OSI-774)-induced inhibition of transitional cell carcinoma of bladder cell line growth is enhanced by interferon-alpha. BJU Int. 2007;99:1539–45.
Yang JL, Qu XJ, Russell PJ, Goldstein D. Interferon-alpha promotes the anti-proliferative effect of Erlotinib (OSI-774) on human colon cancer cell lines. Cancer Lett. 2005;225:61–74.
Bruzzese F, Di Gennaro E, Avallone A, Pepe S, Arra C, Caraglia M, et al. Synergistic antitumor activity of epidermal growth factor receptor tyrosine kinase inhibitor gefitinib and IFN-alpha in head and neck cancer cells in vitro and in vivo. Clin Cancer Res. 2006;12:617–25.
Cheon H, Borden EC, Stark GR. Interferons and their stimulated genes in the tumor microenvironment. Semin Oncol. 2014;41:156–73.
Fensterl V, Sen GC. The ISG56/IFIT1 gene family. J Interferon Cytokine Res. 2011;31:71–8.
Diamond MS. IFIT1: A dual sensor and effector molecule that detects non-2′-O methylated viral RNA and inhibits its translation. Cytokine Growth Factor Rev. 2014;25:543–50.
Fensterl V, Sen GC. Interferon-induced Ifit proteins: their role in viral pathogenesis. J Virol. 2015;89:2462–8.
Zhang JF, Chen Y, Lin GS, Zhang JD, Tang WL, Huang JH, et al. High IFIT1 expression predicts improved clinical outcome, and IFIT1 along with MGMT more accurately predicts prognosis in newly diagnosed glioblastoma. Hum Pathol. 2016;52:136–44.
Zhao Y, Altendorf-Hofmann A, Pozios I, Camaj P, Daberitz T, Wang X, et al. Elevated interferon-induced protein with tetratricopeptide repeats 3 (IFIT3) is a poor prognostic marker in pancreatic ductal adenocarcinoma. J Cancer Res Clin Oncol. 2017;143:1061–8.
Niess H, Camaj P, Mair R, Renner A, Zhao Y, Jackel C, et al. Overexpression of IFN-induced protein with tetratricopeptide repeats 3 (IFIT3) in pancreatic cancer: cellular “pseudoinflammation” contributing to an aggressive phenotype. Oncotarget. 2015;6:3306–18.
Lai KC, Chang KW, Liu CJ, Kao SY, Lee TC. IFN-induced protein with tetratricopeptide repeats 2 inhibits migration activity and increases survival of oral squamous cell carcinoma. Mol Cancer Res. 2008;6:1431–9.
Lai KC, Liu CJ, Chang KW, Lee TC. Depleting IFIT2 mediates atypical PKC signaling to enhance the migration and metastatic activity of oral squamous cell carcinoma cells. Oncogene. 2013;32:3686–97.
Danish HH, Goyal S, Taunk NK, Wu H, Moran MS, Haffty BG. Interferon-induced protein with tetratricopeptide repeats 1 (IFIT1) as a prognostic marker for local control in T1-2 N0 breast cancer treated with breast-conserving surgery and radiation therapy (BCS+RT). Breast J. 2013;19:231–9.
Yang Y, Zhou Y, Hou J, Bai C, Li Z, Fan J. et al. Hepatic IFIT3 predicts interferon-alpha therapeutic response in patients of hepatocellular carcinoma. Hepatology. 2017;66:152–66.
Shield KD, Ferlay J, Jemal A, Sankaranarayanan R, Chaturvedi AK, Bray F, et al. The global incidence of lip, oral cavity, and pharyngeal cancers by subsite in 2012. CA Cancer J Clin. 2017;67:51–64.
Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–86.
Krishna Rao SV, Mejia G, Roberts-Thomson K, Logan R. Epidemiology of oral cancer in Asia in the past decade—an update (2000-12). Asian Pac J Cancer Prev. 2013;14:5567–77.
Chiang CJ, Lo WC, Yang YW, You SL, Chen CJ, Lai MS. Incidence and survival of adult cancer patients in Taiwan, 2002–2012. J Formos Med Assoc. 2016;115:1076–88.
Rivera C, Venegas B. Histological and molecular aspects of oral squamous cell carcinoma (Review). Oncol Lett. 2014;8:7–11.
Shingaki S, Takada M, Sasai K, Bibi R, Kobayashi T, Nomura T, et al. Impact of lymph node metastasis on the pattern of failure and survival in oral carcinomas. Am J Surg. 2003;185:278–84.
Lang J, Gao L, Guo Y, Zhao C, Zhang C. Comprehensive treatment of squamous cell cancer of head and neck: Chinese expert consensus 2013. Future Oncol. 2014;10:1635–48.
Bagan J, Sarrion G, Jimenez Y. Oral cancer: clinical features. Oral Oncol. 2010;46:414–7.
Chen IH, Chang JT, Liao CT, Wang HM, Hsieh LL, Cheng AJ. Prognostic significance of EGFR and Her-2 in oral cavity cancer in betel quid prevalent area cancer prognosis. Br J Cancer. 2003;89:681–6.
Bossi P, Resteghini C, Paielli N, Licitra L, Pilotti S, Perrone F. Prognostic and predictive value of EGFR in head and neck squamous cell carcinoma. Oncotarget. 2016;7:74362–79.
Jimenez L, Jayakar SK, Ow TJ, Segall JE. Mechanisms of invasion in head and neck cancer. Arch Pathol Lab Med. 2015;139:1334–48.
Vermorken JB, Trigo J, Hitt R, Koralewski P, Diaz-Rubio E, Rolland F, et al. Open-label, uncontrolled, multicenter phase II study to evaluate the efficacy and toxicity of cetuximab as a single agent in patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck who failed to respond to platinum-based therapy. J Clin Oncol. 2007;25:2171–7.
Moreira J, Tobias A, O’Brien MP, Agulnik M. Targeted therapy in head and neck cancer: an update on current clinical developments in epidermal growth factor receptor-targeted therapy and immunotherapies. Drugs. 2017;77:843–57.
Huang S-F, Chien H-T, Cheng S-D, Chuang W-Y, Liao C-T, Wang H-M. EGFR copy number alterations in primary tumors, metastatic lymph nodes, and recurrent and multiple primary tumors in oral cavity squamous cell carcinoma. BMC Cancer. 2017;17:592.
Kirby AM, A’Hern RP, D’Ambrosio C, Tanay M, Syrigos KN, Rogers SJ, et al. Gefitinib (ZD1839, Iressa™) as palliative treatment in recurrent or metastatic head and neck cancer. Br J Cancer. 2006;94:631–6.
Grandal MV, Madshus IH. Epidermal growth factor receptor and cancer: control of oncogenic signalling by endocytosis. J Cell Mol Med. 2008;12:1527–34.
Murphy JE, Padilla BE, Hasdemir B, Cottrell GS, Bunnett NW. Endosomes: a legitimate platform for the signaling train. Proc Natl Acad Sci USA. 2009;106:17615–22.
Mosesson Y, Mills GB, Yarden Y. Derailed endocytosis: an emerging feature of cancer. Nat Rev Cancer. 2008;8:835.
Schmid SL. Reciprocal regulation of signaling and endocytosis: implications for the evolving cancer cell. J Cell Biol. 2017;216:2623–32.
D’Andrea LD, Regan L. TPR proteins: the versatile helix. Trends Biochem Sci. 2003;28:655–62.
Szklarczyk D, Morris JH, Cook H, Kuhn M, Wyder S, Simonovic M, et al. The STRING database in 2017: quality-controlled protein–protein association networks, made broadly accessible. Nucleic Acids Res. 2017;45:D362–8.
de Graauw M, Cao L, Winkel L, van Miltenburg MH, le Devedec SE, Klop M, et al. Annexin A2 depletion delays EGFR endocytic trafficking via cofilin activation and enhances EGFR signaling and metastasis formation. Oncogene. 2014;33:2610–9.
Staquicini DI, Rangel R, Guzman-Rojas L, Staquicini FI, Dobroff AS, Tarleton CA, et al. Intracellular targeting of annexin A2 inhibits tumor cell adhesion, migration, and in vivo grafting. Sci Rep. 2017;7:4243.
Christiansen JJ, Rajasekaran AK. Reassessing epithelial to mesenchymal transition as a prerequisite for carcinoma invasion and metastasis. Cancer Res. 2006;66:8319–26.
Zuo JH, Zhu W, Li MY, Li XH, Yi H, Zeng GQ, et al. Activation of EGFR promotes squamous carcinoma SCC10A cell migration and invasion via inducing EMT-like phenotype change and MMP-9-mediated degradation of E-cadherin. J Cell Biochem. 2011;112:2508–17.
Hong KO, Kim JH, Hong JS, Yoon HJ, Lee JI, Hong SP, et al. Inhibition of Akt activity induces the mesenchymal-to-epithelial reverting transition with restoring E-cadherin expression in KB and KOSCC-25B oral squamous cell carcinoma cells. J Exp Clin Cancer Res. 2009;28:28.
Yoon H, Dehart JP, Murphy JM, Lim, S-TS. Understanding the roles of FAK in cancer: inhibitors, genetic models, and new insights. J Histochem Cytochem. 2015;63:114–28.
Sieg DJ, Hauck CR, Ilic D, Klingbeil CK, Schaefer E, Damsky CH, et al. FAK integrates growth-factor and integrin signals to promote cell migration. Nat Cell Biol. 2000;2:249–56.
Krisanaprakornkit S, Iamaroon A. Epithelial-mesenchymal transition in oral squamous cell carcinoma. ISRN Oncol. 2012;2012:10.
Yilmaz M, Christofori G. EMT, the cytoskeleton, and cancer cell invasion. Cancer Metastas Rev. 2009;28:15–33.
Moon C, Chae YK, Lee J. Targeting epidermal growth factor receptor in head and neck cancer: lessons learned from cetuximab. Exp Biol Med. 2010;235:907–20.
Mehra R, Serebriiskii IG, Dunbrack RL Jr., Robinson MK, Burtness B, et al. Protein-intrinsic and signaling network-based sources of resistance to EGFR- and ErbB family-targeted therapies in head and neck cancer. Drug Resist Updat. 2011;14:260–79.
Ribeiro FA, Noguti J, Oshima CT, Ribeiro DA. Effective targeting of the epidermal growth factor receptor (EGFR) for treating oral cancer: a promising approach. Anticancer Res. 2014;34:1547–52.
Perea S, Oppenheimer D, Amador M, Cusati G, Baker S, Takimoto C, et al. Genotypic bases of EGFR inhibitors pharmacological actions. J Clin Oncol. 2004;22:3005–3005.
Ono M, Kuwano M. Molecular mechanisms of epidermal growth factor receptor (EGFR) activation and response to gefitinib and other EGFR-targeting drugs. Clin Cancer Res. 2006;12:7242–51.
Grewal T, Enrich C. Annexins—modulators of EGF receptor signalling and trafficking. Cell Signal. 2009;21:847–58.
Shetty PK, Thamake SI, Biswas S, Johansson SL, Vishwanatha JK. Reciprocal regulation of annexin A2 and EGFR with Her-2 in Her-2 negative and herceptin-resistant breast cancer. PLoS ONE. 2012;7:e44299.
Blatch GL, Lassle M. The tetratricopeptide repeat: a structural motif mediating protein-protein interactions. Bioessay News Rev Mol Cell Dev Biol. 1999;21:932–9.
Gerke V, Creutz CE, Moss SE. Annexins: linking Ca2+signalling to membrane dynamics. Nat Rev Mol Cell Biol. 2005;6:449–61.
Bharadwaj A, Bydoun M, Holloway R, Waisman D. Annexin A2 heterotetramer: structure and function. Int J Mol Sci. 2013;14:6259–305.
Futter CE, White IJ. Annexins and endocytosis. Traffic. 2007;8:951–8.
Fang YT, Lin CF, Wang CY, Anderson R, Lin YS. Interferon-gamma stimulates p11-dependent surface expression of annexin A2 in lung epithelial cells to enhance phagocytosis. J Cell Physiol. 2012;227:2775–87.
Hayes MJ, Shao D, Bailly M, Moss SE. Regulation of actin dynamics by annexin 2. EMBO J. 2006;25:1816–26.
Lorusso A, Covino C, Priori G, Bachi A, Meldolesi J, Chieregatti E. Annexin2 coating the surface of enlargeosomes is needed for their regulated exocytosis. EMBO J. 2006;25:5443–56.
Morel E, Gruenberg J. Annexin A2 binding to endosomes and functions in endosomal transport are regulated by tyrosine 23 phosphorylation. J Biol Chem. 2009;284:1604–11.
Kapoor C, Vaidya S, Wadhwan V, Malik S. Lymph node metastasis: a bearing on prognosis in squamous cell carcinoma. Indian J Cancer. 2015;52:417–24.
Matsushita Y, Yanamoto S, Takahashi H, Yamada S, Naruse T, Sakamoto Y, et al. A clinicopathological study of perineural invasion and vascular invasion in oral tongue squamous cell carcinoma. Int J Oral Maxillofac Surg. 2015;44:543–8.
Zhou X, Temam S, Oh M, Pungpravat N, Huang B-L, Mao L, et al. Global expression-based classification of lymph node metastasis and extracapsular spread of oral tongue squamous cell carcinoma. Neoplasia. 2006;8:925–32.
Acknowledgements
We are thankful to Dr. Fann Cathy S.-J, and Ms. Jenny Chang, Institute of Biomedical Sciences, for their help in clinical data analysis. We are grateful for the excellent technical services provided by Dr. Fu-An Li, Proteomic Core Laboratories, Taiwan Mouse Clinic, and Pathology Core laboratories, and Institute of Biomedical Sciences, Academia Sinica, Taipei, Taiwan.
Funding
This work was supported by grants from the Ministry of Science and Technology (NSC 101-2321-B-001-044, NSC 102-2321-B-001-032, MOST 103-2321-B-001-019, and MOST 104-2320-B-001-008), Taiwan (T.C. Lee).
Author contributions
VKP and TCL designed the research. VKP performed cellular, molecular, and biochemical experiments. VKP and HBP performed animal experiments. VKP, AHY and HBP performed immunohistochemical assays. VKP, MMW, HBP, KWC and CJL analyzed the data. CJL provided the patient’s information and tumor samples. VKP wrote the manuscript. VKP, HBP and TCL revised the manuscript. CJL and TCL supervised the study. All authors read and approved the manuscript.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Pidugu, V.K., Wu, MM., Yen, AH. et al. IFIT1 and IFIT3 promote oral squamous cell carcinoma metastasis and contribute to the anti-tumor effect of gefitinib via enhancing p-EGFR recycling. Oncogene 38, 3232–3247 (2019). https://doi.org/10.1038/s41388-018-0662-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41388-018-0662-9
This article is cited by
-
Targeting inflammation as cancer therapy
Journal of Hematology & Oncology (2024)
-
IFIT1 modulates the proliferation, migration and invasion of pancreatic cancer cells via Wnt/β-catenin signaling
Cellular Oncology (2024)
-
ETV7 promotes colorectal cancer progression through upregulation of IFIT3
Functional & Integrative Genomics (2024)
-
The diagnostic/prognostic roles and biological function of the IFIT family members in acute myeloid leukemia
BMC Medical Genomics (2023)
-
Kruppel-like factor 13 acts as a tumor suppressor in thyroid carcinoma by downregulating IFIT1
Biology Direct (2023)