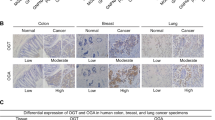
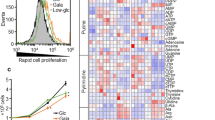
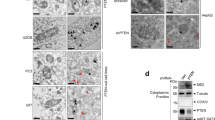
Abstract
Increased glucose consumption is a hallmark of cancer cells. The increased consumption and subsequent metabolism of glucose during proliferation creates the need for a constant supply of NAD, a co-factor in glycolysis. Regeneration of the NAD required to support enhanced glycolysis has been attributed to the terminal glycolytic enzyme, lactate dehydrogenase (LDH). However, loss of glucose carbons to biosynthetic pathways early in glycolysis reduces the carbon supply to LDH. Thus, alternative routes for NAD regeneration must exist to support the increased glycolytic rate while allowing for the diversion of glucose to generate biomass and support proliferation. Here we demonstrate, using a variety of cancer cell lines as well as activated primary T cells, that cytosolic malate dehydrogenase 1 (MDH1) is an alternative to LDH as a supplier of NAD. Moreover, our results indicate that MDH1 generates malate with carbons derived from glutamine, thus enabling utilization of glucose carbons for glycolysis and for biomass. Amplification of MDH1 occurs at an impressive frequency in human tumors and correlates with poor prognosis. Together, our findings suggest that proliferating cells rely on both MDH1 and LDH to replenish cytosolic NAD, and that therapies designed at targeting glycolysis must consider both dehydrogenases.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Lunt SY, Vander Heiden MG . Aerobic glycolysis: meeting the metabolic requirements of cell proliferation. Annu Rev Cell Dev Biol 2011; 27: 441–464.
Goldman RD, Kaplan NO, Hall TC . Lactic dehydrogenase in human neoplastic tissues. Cancer Res 1964; 24: 389–399.
Liberti MV, Locasale JW . The Warburg effect: how does it benefit cancer cells? Trends Biochem Sci 2016; 41: 211–218.
Lowman XH, McDonnell MA, Kosloske A, Odumade OA, Jenness C, Karim CB et al. The proapoptotic function of Noxa in human leukemia cells is regulated by the kinase Cdk5 and by glucose. Mol Cell 2010; 40: 823–833.
Hirschhaeuser F, Sattler UG, Mueller-Klieser W . Lactate: a metabolic key player in cancer. Cancer Res 2011; 71: 6921–6925.
Lamesch P, Li N, Milstein S, Fan C, Hao T, Szabo G et al. hORFeome v3.1: a resource of human open reading frames representing over 10,000 human genes. Genomics 2007; 89: 307–315.
Yang Z, Savchenko A, Yakunin A, Zhang R, Edwards A, Arrowsmith C et al. Aspartate dehydrogenase, a novel enzyme identified from structural and functional studies of TM1643. J Biol Chem 2003; 278: 8804–8808.
Menzies KJ, Zhang H, Katsyuba E, Auwerx J . Protein acetylation in metabolism - metabolites and cofactors. Nat Rev Endocrinol 2016; 12: 43–60.
Carr EL, Kelman A, Wu GS, Gopaul R, Senkevitch E, Aghvanyan A et al. Glutamine uptake and metabolism are coordinately regulated by ERK/MAPK during T lymphocyte activation. J Immunol 2010; 185: 1037–1044.
Frauwirth KA, Riley JL, Harris MH, Parry RV, Rathmell JC, Plas DR et al. The CD28 signaling pathway regulates glucose metabolism. Immunity 2002; 16: 769–777.
Pike Winer LS, Wu M . Rapid analysis of glycolytic and oxidative substrate flux of cancer cells in a microplate. PLoS One 2014; 9: e109916.
Goldberg EB, Colowick SP . The role of glycolysis in the growth of tumor cells. 3. Lactic dehydrogenase as the site of action of oxamate on the growth of cultured cells. J Biol Chem 1965; 240: 2786–2790.
Calvo MN, Bartrons R, Castano E, Perales JC, Navarro-Sabate A, Manzano A . PFKFB3 gene silencing decreases glycolysis, induces cell-cycle delay and inhibits anchorage-independent growth in HeLa cells. FEBS Lett 2006; 580: 3308–3314.
Birsoy K, Wang T, Chen WW, Freinkman E, Abu-Remaileh M, Sabatini DM . An essential role of the mitochondrial electron transport chain in cell proliferation is to enable aspartate synthesis. Cell 2015; 162: 540–551.
Gui DY, Sullivan LB, Luengo A, Hosios AM, Bush LN, Gitego N et al. Environment dictates dependence on mitochondrial complex I for NAD+ and aspartate production and determines cancer cell sensitivity to metformin. Cell Metab 2016; 24: 716–727.
Sullivan LB, Gui DY, Hosios AM, Bush LN, Freinkman E, Vander Heiden MG . Supporting aspartate biosynthesis is an essential function of respiration in proliferating cells. Cell 2015; 162: 552–563.
Billiard J, Dennison JB, Briand J, Annan RS, Chai D, Colon M et al. Quinoline 3-sulfonamides inhibit lactate dehydrogenase A and reverse aerobic glycolysis in cancer cells. Cancer Metab 2013; 1: 19.
Augoff K, Hryniewicz-Jankowska A, Tabola R . Lactate dehydrogenase 5: an old friend and a new hope in the war on cancer. Cancer Lett 2015; 358: 1–7.
Rani R, Kumar V . Recent update on human lactate dehydrogenase enzyme 5 (hLDH5) inhibitors: a promising approach for cancer chemotherapy. J Med Chem 2015; 59: 487–496.
Xie H, Hanai J, Ren JG, Kats L, Burgess K, Bhargava P et al. Targeting lactate dehydrogenase—a inhibits tumorigenesis and tumor progression in mouse models of lung cancer and impacts tumor-initiating cells. Cell Metab 2014; 19: 795–809.
Gao J, Aksoy BA, Dogrusoz U, Dresdner G, Gross B, Sumer SO et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal 2013; 6: pl1.
Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov 2012; 2: 401–404.
Liu PP, Liao J, Tang ZJ, Wu WJ, Yang J, Zeng ZL et al. Metabolic regulation of cancer cell side population by glucose through activation of the Akt pathway. Cell Death Differ 2014; 21: 124–135.
Lum JJ, Bui T, Gruber M, Gordan JD, DeBerardinis RJ, Covello KL et al. The transcription factor HIF-1alpha plays a critical role in the growth factor-dependent regulation of both aerobic and anaerobic glycolysis. Genes Dev 2007; 21: 1037–1049.
Wise DR, Ward PS, Shay JE, Cross JR, Gruber JJ, Sachdeva UM et al. Hypoxia promotes isocitrate dehydrogenase-dependent carboxylation of alpha-ketoglutarate to citrate to support cell growth and viability. Proc Natl Acad Sci USA 2011; 108: 19611–19616.
Fendt SM, Bell EL, Keibler MA, Olenchock BA, Mayers JR, Wasylenko TM et al. Reductive glutamine metabolism is a function of the alpha-ketoglutarate to citrate ratio in cells. Nat Commun 2013; 4: 2236.
Fan J, Ye J, Kamphorst JJ, Shlomi T, Thompson CB, Rabinowitz JD . Quantitative flux analysis reveals folate-dependent NADPH production. Nature 2014; 510: 298–302.
Lewis CA, Parker SJ, Fiske BP, McCloskey D, Gui DY, Green CR et al. Tracing compartmentalized NADPH metabolism in the cytosol and mitochondria of mammalian cells. Mol Cell 2014; 55: 253–263.
Liu L, Shah S, Fan J, Park JO, Wellen KE, Rabinowitz JD . Malic enzyme tracers reveal hypoxia-induced switch in adipocyte NADPH pathway usage. Nat Chem Biol 2016; 12: 345–352.
Wang YP, Zhou W, Wang J, Huang X, Zuo Y, Wang TS et al. Arginine methylation of MDH1 by CARM1 inhibits glutamine metabolism and suppresses pancreatic cancer. Mol Cell 2016; 64: 673–687.
Christofk HR, Vander Heiden MG, Harris MH, Ramanathan A, Gerszten RE, Wei R et al. The M2 splice isoform of pyruvate kinase is important for cancer metabolism and tumour growth. Nature 2008; 452: 230–233.
Lee SM, Kim JH, Cho EJ, Youn HD . A nucleocytoplasmic malate dehydrogenase regulates p53 transcriptional activity in response to metabolic stress. Cell Death Differ 2009; 16: 738–748.
Lorenz MA, Burant CF, Kennedy RT . Reducing time and increasing sensitivity in sample preparation for adherent mammalian cell metabolomics. Anal Chem 2011; 83: 3406–3414.
Acknowledgements
We thank Drs David Sabatini and Kivanc Birsoy for the MDH1 KO Jurkat cells, and Hong-Duk Youn for the MDH1 construct. We would also like to thank Todd Rappe and University of Minnesota Nuclear Magnetic Resonance Core for help with nuclear magnetic resonance analysis. We are grateful to Dr David Bernlohr, Michael Downey, Yan Yan and Dr Jenna Benson for stimulating discussions. We also thank Drs Lucas Sullivan and Sandra Armstrong for valuable suggestions. This study was supported by a Seed Grant and an Infrastructure grant from the University of Minnesota Academic Health Center (to AK), National Institutes of Health (NIH) grant R01 CA157971, P/F grant, 3002751553, through the NIH-funded RCMC in Michigan (to AK), and by T32 training fellowship, CA009138, and F31 award, CA177119 (to EAH). The study utilized Metabolomics Core Services supported by grant U24 DK097153 of NIH Common Funds Project to the University of Michigan.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Hanse, E., Ruan, C., Kachman, M. et al. Cytosolic malate dehydrogenase activity helps support glycolysis in actively proliferating cells and cancer. Oncogene 36, 3915–3924 (2017). https://doi.org/10.1038/onc.2017.36
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2017.36
This article is cited by
-
Metabolic priming by multiple enzyme systems supports glycolysis, HIF1α stabilisation, and human cancer cell survival in early hypoxia
The EMBO Journal (2024)
-
Tat-malate dehydrogenase fusion protein protects neurons from oxidative and ischemic damage by reduction of reactive oxygen species and modulation of glutathione redox system
Scientific Reports (2023)
-
CD40 signal rewires fatty acid and glutamine metabolism for stimulating macrophage anti-tumorigenic functions
Nature Immunology (2023)
-
Attenuates of NAD+ impair BMSC osteogenesis and fracture repair through OXPHOS
Stem Cell Research & Therapy (2022)
-
A glycolysis-based three-gene signature predicts survival in patients with lung squamous cell carcinoma
BMC Cancer (2021)