Abstract
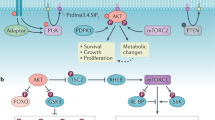
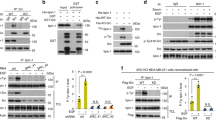
An enhanced capacity for de novo lipid synthesis is a metabolic feature of most cancer cells that distinguishes them from their cells of origin. However, the mechanisms through which oncogenes alter lipid metabolism are poorly understood. We find that expression of oncogenic PI3K (H1047R) or K-Ras (G12V) in breast epithelial cells is sufficient to induce de novo lipogenesis, and this occurs through the convergent activation of the mechanistic target of rapamycin complex 1 (mTORC1) downstream of these common oncogenes. Oncogenic stimulation of mTORC1 signaling in this isogenic setting or a panel of eight breast cancer cell lines leads to activation of the sterol regulatory element-binding proteins (SREBP1 and SREBP2) that are required for oncogene-induced lipid synthesis. The SREBPs are also required for the growth factor-independent growth and proliferation of oncogene-expressing cells. Finally, we find that elevated mTORC1 signaling is associated with increased mRNA and protein levels of canonical SREBP targets in primary human breast cancer samples. These data suggest that the mTORC1/SREBP pathway is a major mechanism through which common oncogenic signaling events induce de novo lipid synthesis to promote aberrant growth and proliferation of cancer cells.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Medes G, Thomas A, Weinhouse S . Metabolism of neoplastic tissue. IV. A study of lipid synthesis in neoplastic tissue slices in vitro. Cancer Res 1953; 13: 27–29.
Menendez JA, Lupu R . Fatty acid synthase and the lipogenic phenotype in cancer pathogenesis. Nat Rev Cancer 2007; 7: 763–777.
Santos CR, Schulze A . Lipid metabolism in cancer. FEBS J 2012; 279: 2610–2623.
Kuhajda FP, Jennert K, Wood FD, Hennigart RA, Jacobs LB, Dick JD et al. Fatty acid synthesis : a potential selective target for antineoplastic therapy. Proc Natl Acad Sci USA 1994; 91: 6379–6383.
Li J, Ding S, Habib N, Fermor B, Wood C, Gilmour R . Partial characterization of a cDNA for human stearoyl-CoA desaturase and changes in its mRNA expression in some normal and malignant tissues. Int J Cancer 1994; 57: 348–352.
Horton JD, Goldstein JL, Brown MS . SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver. J Clin Invest 2002; 109: 1125–1131.
Wang X, Sato R, Brown MS, Hua X, Goldsteln JL . SREBP-1, a membrane-bound transcription factor released by sterol-regulated proteolysis. Cell 1994; 77: 53–62.
Hua X, Sakai J, Brown MS, Goldstein JL . Regulated cleavage of sterol regulatory element binding proteins requires sequences on both sides of the endoplasmic reticulum membrane. J Biol Chem 1996; 271: 10379–10384.
Sakai J, Duncan EA, Rawson RB, Hua X, Brown MS, Goldstein JL . Sterol-regulated release of SREBP-2 from cell membranes requires two sequential cleavages, one within a transmembrane segment. Cell 1996; 85: 1037–1046.
Goldstein JL, DeBose-Boyd RA, Brown MS . Protein sensors for membrane sterols. Cell 2006; 124: 35–46.
Jeon T, Osborne TF . SREBPs: metabolic integrators in physiology and metabolism. Trends Endocrinol Metab 2011; 23: 65–72.
Ricoult SJH, Manning BD . The multifaceted role of mTORC1 in the control of lipid metabolism. EMBO Rep 2013; 14: 242–251.
Düvel K, Yecies JL, Menon S, Raman P, Lipovsky AI, Souza AL et al. Activation of a metabolic gene regulatory network downstream of mTOR complex 1. Mol Cell 2010; 39: 171–183.
Porstmann T, Santos CR, Griffiths B, Cully M, Wu M, Leevers S et al. SREBP activity is regulated by mTORC1 and contributes to Akt-dependent cell growth. Cell Metab 2008; 8: 224–236.
Li S, Brown MS, Goldstein JL . Bifurcation of insulin signaling pathway in rat liver: mTORC1 required for stimulation of lipogenesis, but not inhibition of gluconeogenesis. Proc Natl Acad Sci USA 2010; 107: 3441–3446.
Yecies JL, Zhang HH, Menon S, Liu S, Yecies D, Lipovsky AI et al. Akt stimulates hepatic SREBP1c and lipogenesis through parallel mTORC1-dependent and independent pathways. Cell Metab 2011; 14: 21–32.
Li S, Ogawa W, Emi A, Hayashi K, Senga Y, Nomura K et al. Role of S6K1 in regulation of SREBP1c expression in the liver. Biochem Biophys Res Commun 2011; 412: 197–202.
Wang BT, Ducker GS, Barczak AJ, Barbeau R, Erle DJ, Shokat KM . The mammalian target of rapamycin regulates cholesterol biosynthetic gene expression and exhibits a rapamycin-resistant transcriptional profile. Proc Natl Acad Sci USA 2011; 108: 15201–15206.
Liu X, Yuan H, Niu Y, Niu W, Fu L . The role of AMPK/mTOR/S6K1 signaling axis in mediating the physiological process of exercise-induced insulin sensitization in skeletal muscle of C57BL/6 mice. Biochim Biophys Acta 2012; 1822: 1716–1726.
Owen JL, Zhang Y, Bae S-H, Farooqi MS, Liang G, Hammer RE et al. Insulin stimulation of SREBP-1c processing in transgenic rat hepatocytes requires p70 S6-kinase. Proc Natl Acad Sci USA 2012; 109: 16184–16189.
Luyimbazi D, Akcakanat A, McAuliffe PF, Zhang L, Singh G, Gonzalez-Angulo AM et al. Rapamycin regulates stearoyl CoA desaturase 1 expression in breast cancer. Mol Cancer Ther 2010; 9: 2770–2784.
Peterson TR, Sengupta SS, Harris TE, Carmack AE, Kang SA, Balderas E et al. mTOR complex 1 regulates lipin 1 localization to control the SREBP pathway. Cell 2011; 146: 408–420.
Feldman ME, Apsel B, Uotila A, Loewith R, Knight ZA, Ruggero D et al. Active-site inhibitors of mTOR target rapamycin-resistant outputs of mTORC1 and mTORC2. PLoS Biol 2009; 7: e1000038.
Thoreen CC, Kang SA, Chang JW, Liu Q, Zhang J, Gao Y et al. An ATP-competitive mammalian target of rapamycin inhibitor reveals rapamycin-resistant functions of mTORC1. J Biol Chem 2009; 284: 8023–8032.
Dibble CC, Elis W, Menon S, Qin W, Klekota J, Asara JM et al. TBC1D7 is a third subunit of the TSC1-TSC2 complex upstream of mTORC1. Mol Cell 2012; 47: 535–546.
Dibble CC, Manning BD . Signal integration by mTORC1 coordinates nutrient input with biosynthetic output. Nat Cell Biol 2013; 15: 555–564.
Huang J, Manning BD . The TSC1–TSC2 complex: a molecular switchboard controlling cell growth. Biochem J 2008; 412: 179–190.
Crino PB, Nathanson KL, Henske EP . The tuberous sclerosis complex. N Engl J Med 2006; 355: 1345–1356.
Menon S, Manning BD . Common corruption of the mTOR signaling network in human tumors. Oncogene 2008; 27: S43–S51.
Manning BD, Tee AR, Logsdon MN, Blenis J, Cantley LC . Identification of the tuberous sclerosis complex-2 tumor suppressor gene product tuberin as a target of the phosphoinositide 3-kinase/akt pathway. Mol Cell 2002; 10: 151–162.
Inoki K, Li Y, Zhu T, Wu J, Guan K . TSC2 is phosphorylated and inhibited by Akt and suppresses mTOR signalling. Nat Cell Biol 2002; 4: 648–657.
Johannessen CM, Reczek EE, James MF, Brems H, Legius E, Cichowski K . The NF1 tumor suppressor critically regulates TSC2 and mTOR. Proc Natl Acad Sci USA 2005; 102: 8573–8578.
Ma L, Chen Z, Erdjument-Bromage H, Tempst P, Pandolfi PP . Phosphorylation and functional inactivation of TSC2 by Erk implications for tuberous sclerosis and cancer pathogenesis. Cell 2005; 121: 179–193.
Roux PP, Ballif BA, Anjum R, Gygi SP, Blenis J . Tumor-promoting phorbol esters and activated Ras inactivate the tuberous sclerosis tumor suppressor complex via p90 ribosomal S6 kinase. Proc Natl Acad Sci USA 2004; 101: 13489–13494.
Tweto J, Liberati M, Larrabee A . Protein turnover and 4’-phosphopantetheine exchange in rat liver fatty acid synthetase. J Biol Chem 1971; 246: 2468–2471.
Nakanishi S, Numa S . Purification of rat liver acetyl coenzyme A carboxylase and immunochemical studies on its synthesis and degradation. Eur J Biochem 1970; 16: 161–173.
Griffiths B, Lewis CA, Bensaad K, Ros S, Zhang Q, Ferber EC et al. Sterol regulatory element binding protein-dependent regulation of lipid synthesis supports cell survival and tumor growth. Cancer Metab 2013; 1: 3.
Kidani Y, Elsaesser H, Hock MB, Vergnes L, Williams KJ, Argus JP et al. Sterol regulatory element–binding proteins are essential for the metabolic programming of effector T cells and adaptive immunity. Nat Immunol 2013; 14: 489–499.
Amemiya-Kudo M, Shimano H, Yoshikawa T, Yahagi N, Hasty AH, Okazaki H et al. Promoter analysis of the mouse sterol regulatory element-binding protein-1c gene. J Biol Chem 2000; 275: 31078–31085.
Cancer Genome Atlas Network. Comprehensive molecular portraits of human breast tumours. Nature 2012; 490: 61–70.
Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov 2012; 2: 401–404.
Gao J, Aksoy BA, Dogrusoz U, Dresdner G, Gross B, Sumer SO et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal 2013; 6: pl1.
Howell JJ, Ricoult SJH, Ben-Sahra I, Manning BD . A growing role for mTOR in promoting anabolic metabolism. Biochem Soc Trans 2013; 41: 906–912.
Porstmann T, Griffiths B, Chung Y-L, Delpuech O, Griffiths JR, Downward J et al. PKB/Akt induces transcription of enzymes involved in cholesterol and fatty acid biosynthesis via activation of SREBP. Oncogene 2005; 24: 6465–6481.
Guo D, Prins RRM, Dang J, Kuga D, Iwanami A, Soto H et al. EGFR signaling through an Akt-SREBP-1-dependent, rapamycin-resistant pathway sensitizes glioblastomas to antilipogenic therapy. Sci Signal 2009; 2: ra82.
Krycer JRJ, Phan L, Brown AJA . A key regulator of cholesterol homeostasis, SREBP-2, can be targeted in prostate cancer cells with natural products. Biochem J 2012; 446: 191–201.
Williams KJ, Argus JP, Zhu Y, Wilks MQ, Marbois BN, York AG et al. An essential requirement for the SCAP/SREBP signaling axis to protect cancer cells from lipotoxicity. Cancer Res 2013; 73: 2850–2862.
Shimano H, Shimomura I, Hammer RE, Herz J, Goldstein JL, Brown MS et al. Elevated levels of SREBP-2 and cholesterol synthesis in livers of mice homozygous for a targeted disruption of the SREBP-1 gene. J Clin Invest 1997; 100: 2115–2124.
Hatzivassiliou G, Zhao F, Bauer DE, Andreadis C, Shaw AN, Dhanak D et al. ATP citrate lyase inhibition can suppress tumor cell growth. Cancer Cell 2005; 8: 311–321.
Migita T, Narita T, Nomura K, Miyagi E, Inazuka F, Matsuura M et al. ATP citrate lyase: activation and therapeutic implications in non-small cell lung cancer. Cancer Res 2008; 68: 8547–8554.
Fritz V, Benfodda Z, Rodier G, Henriquet C, Iborra F, Avancès C et al. Abrogation of de novo lipogenesis by stearoyl-CoA desaturase 1 inhibition interferes with oncogenic signaling and blocks prostate cancer progression in mice. Mol Cancer Ther 2010; 9: 1740–1754.
Roongta UV, Pabalan JG, Wang X, Ryseck R-P, Fargnoli J, Henley BJ et al. Cancer cell dependence on unsaturated fatty acids implicates stearoyl-CoA desaturase as a target for cancer therapy. Mol Cancer Res 2011; 9: 1551–1561.
Minville-Walz M, Pierre A-S, Pichon L, Bellenger S, Fèvre C, Bellenger J et al. Inhibition of stearoyl-CoA desaturase 1 expression induces CHOP-dependent cell death in human cancer cells. PLoS One 2010; 5: e14363.
Young RM, Ackerman D, Quinn ZL, Mancuso A, Gruber M, Liu L et al. Dysregulated mTORC1 renders cells critically dependent on desaturated lipids for survival under tumor-like stress. Genes Dev 2013; 27: 1115–1131.
Freed-Pastor WA, Mizuno H, Zhao X, Langerød A, Moon S-H, Rodriguez-Barrueco R et al. Mutant p53 disrupts mammary tissue architecture via the mevalonate pathway. Cell 2012; 148: 244–258.
Clendening JW, Penn LZ . Targeting tumor cell metabolism with statins. Oncogene 2012; 31: 4967–4978.
Kamphorst JJ, Cross JR, Fan J, de Stanchina E, Mathew R, White EP et al. Hypoxic and Ras-transformed cells support growth by scavenging unsaturated fatty acids from lysophospholipids. Proc Natl Acad Sci USA 2013; 110: 8882–8887.
Morrisett JD, Abdel-Fattah G, Hoogeveen R, Mitchell E, Ballantyne CM, Pownall HJ et al. Effects of sirolimus on plasma lipids, lipoprotein levels, and fatty acid metabolism in renal transplant patients. J Lipid Res 2002; 43: 1170–1180.
Kasiske BL, de Mattos A, Flechner SM, Gallon L, Meier-Kriesche HU, Weir MR et al. Mammalian target of rapamycin inhibitor dyslipidemia in kidney transplant recipients. Am J Transpl 2008; 8: 1384–1392.
Zhang C, Yoon M-S, Chen J . Amino acid-sensing mTOR signaling is involved in modulation of lipolysis by chronic insulin treatment in adipocytes. Am J Physiol Endocrinol Metab 2009; 296: E862–E868.
Chakrabarti P, English T, Shi J, Smas CM, Kandror KV . Mammalian target of rapamycin complex 1 suppresses lipolysis, stimulates lipogenesis, and promotes fat storage. Diabetes 2010; 59: 775–781.
Soliman GA, Acosta-Jaquez HA, Fingar DC . mTORC1 inhibition via rapamycin promotes triacylglycerol lipolysis and release of free fatty acids in 3T3-L1 adipocytes. Lipids 2010; 45: 1089–1100.
Zhao JJ, Liu Z, Wang L, Shin E, Loda MF, Roberts TM . The oncogenic properties of mutant p110alpha and p110beta phosphatidylinositol 3-kinases in human mammary epithelial cells. Proc Natl Acad Sci USA 2005; 102: 18443–18448.
Schneider CA, Rasband WS, Eliceiri KW . NIH Image to ImageJ: 25 years of image analysis. Nat Methods 2012; 9: 671–675.
Neve RM, Chin K, Fridlyand J, Yeh J, Frederick L, Fevr T et al. A collection of breast cancer cell lines for the study of functionally distinct cancer subtypes. Cancer Cell 2006; 10: 515–527.
Acknowledgements
This work was supported in part by NSF predoctoral fellowship DGE-1144152 (to SJHR), a postdoctoral fellowship from the LAM Foundation (to IB-S), a Sanofi Innovation Award (to BDM) and NIH Grants R01-CA181390 and P01-CA120964 (to BDM).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Ricoult, S., Yecies, J., Ben-Sahra, I. et al. Oncogenic PI3K and K-Ras stimulate de novo lipid synthesis through mTORC1 and SREBP. Oncogene 35, 1250–1260 (2016). https://doi.org/10.1038/onc.2015.179
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2015.179
This article is cited by
-
Mutant p53 murine oviductal epithelial cells induce progression of high-grade serous carcinoma and are most sensitive to simvastatin therapy in vitro and in vivo
Journal of Ovarian Research (2023)
-
Glycolysis regulates KRAS plasma membrane localization and function through defined glycosphingolipids
Nature Communications (2023)
-
Lithium Chloride Promotes Milk Protein and Fat Synthesis in Bovine Mammary Epithelial Cells via HIF-1α and β-Catenin Signaling Pathways
Biological Trace Element Research (2023)
-
Ferroptosis: opening up potential targets for gastric cancer treatment
Molecular and Cellular Biochemistry (2023)
-
The Metabolic Landscape of Breast Cancer and Its Therapeutic Implications
Molecular Diagnosis & Therapy (2023)