Abstract
It has emerged recently that exosomes are potential carriers of pro-tumorigenic factors that participate in oncogenesis. However, whether oncogenic transcription factors are transduced by exosomes is unknown. Hypoxia-inducible factor-1α (HIF1α) transcriptionally regulates numerous key aspects of tumor development and progression by promoting a more aggressive tumor phenotype, characterized by increased proliferation and invasiveness coupled with neoangiogenesis. It has been shown that the principal oncoprotein of Epstein–Barr virus (EBV), latent membrane protein 1 (LMP1), drives oncogenic processes and tumor progression of the highly invasive EBV malignancy, nasopharyngeal carcinoma (NPC). We now demonstrate that endogenous HIF1α is detectable in exosomes and that LMP1 significantly increases levels of HIF1α in exosomes. HIF1 recovered from exosomes retains DNA-binding activity and is transcriptionally active in recipient cells after exosome uptake. We also show that treatment of EBV-negative cells with LMP1-exosomes increases migration and invasiveness of NP cell lines in functional assays, which correlates with the phenotype associated with epithelial–mesenchymal transition (EMT). In addition, we provide evidence that HIF1α itself participates in exosome-mediated pro-metastatic effects in recipient cells, as exosome-mediated delivery of active and inactive forms of HIF1α results in reciprocal changes in the expression of E- and N-cadherins associated with EMT. Further, immunohistochemical analysis of NPC tumor tissues revealed direct correlation between protein levels of LMP1 and of the endosome/exosome marker tetraspanin, CD63, which suggests an increase in exosome formation in this EBV-positive malignancy. We hypothesize that exosome-mediated transfer of functional pro-metastatic factors by LMP1-positive NPC cells to surrounding tumor cells promotes cancer progression.
Similar content being viewed by others
Introduction
Nasopharyngeal carcinoma (NPC) is a highly invasive malignancy, and 70–90% of patients present with cervical lymph-node metastasis at the time of initial diagnosis. As the biological behavior of NPC depends on its nodal status, patients with advanced nodal disease are likely to have a poor outcome, and drug resistance may hamper the efficacy of anticancer drugs.1
Virtually, all NPC are infected with Epstein–Barr virus (EBV).2 EBV produces latent infection of NPC cells, which persists in the form of EBV episomes. Occasionally, there is sporadic viral reactivation and lytic infection in a few NPC cells. Generally, Type II latency is maintained, and thus EBV-gene expression is restricted to EBNA1, latent membrane protein 1 (LMP1), LMP2, EBERs and BART-encoded miRNAs.3 The EBV primary oncogene LMP1 is expressed in NPC tumor tissue and has been shown to induce transformation, inhibit differentiation and promote migration of epithelial cells. In addition to an etiological role in EBV malignancies, there is circumstantial evidence to suggest that LMP1 also promotes tumor progression by enhancing expression of invasion and metastasis factors.3 LMP2 also contributes to oncogenesis and tumor maintenance.2
Invasion and metastasis are determinative features in the pathogenesis and progression of malignant neoplasms. The process of metastasis consists of multiple, sequential, selective and interdependent steps. As noted, early metastasis directly to regional lymph nodes is common in NPC. To establish a distant metastatic focus, tumor cells must detach from the primary tumor (suppression of cell-to-cell and cell–matrix adhesion), degrade and invade extracellular matrix, increase cell motility and enter the circulation, where they are arrested in capillary beds and gain entrance to organ parenchyma, proliferate and induce angiogenesis. It is now well established that the processes of invasion and angiogenesis are essential to promote and sustain metastases of both primary and metastatic tumors. Furthermore, the epithelial-to-mesenchymal transition (EMT), characterized by the loss of epithelial characteristics and the gain of mesenchymal attributes in epithelial cells, is clearly associated with pathological processes requiring epithelial cell migration and invasion.4
We identified the type IV collagenase matrix metalloproteinase-9 as a key molecule in the destruction of extracellular matrix that is upregulated by LMP1 via nuclear factor-κB and activator protein-1 signaling pathways. Additionally, LMP1 induces mucin 1 and the membrane crosslinker protein ezrin in early steps of cell detachment. Moreover, LMP1 can induce EMT via Twist or Snail, which coincides with the acquisition of cancer stem-cell properties. Recently, special AT-rich-binding protein 1, a global regulator of chromatin remodeling and gene expression, has been identified as a pro-metastatic effector of LMP1 signaling in EBV-positive NPC. We have also shown that LMP1 induces cyclooxygenase-2 and hypoxia-inducible factor-1α (HIF1α), which have key roles in the induction of vascular endothelial growth factor and finally angiogenesis.5
The transcriptional activator HIF16 is the key mediator of the cellular responses to hypoxia and controls the expression of at least 40 genes that are involved in angiogenesis, invasion and metastasis of cancer. HIF1 consists of two subunits: HIF1α and HIF1β (or ARNT). Both contain basic helix loop helix and PER-ARNT-SIM (PAS) domains in their NH2-terminal regions that mediate heterodimerization and binding to DNA regulatory sequences. These sequences, called hypoxia response elements (HREs), are present in the promoter or enhancer regions of HIF1 target genes. Under normoxic conditions, HIF1α is ubiquitinated by Von Hippel Lindau E3 ligase for proteasome degradation in cytoplasm. Once stabilized, HIF1α translocates to the nucleus guided by a nuclear localization signal present in its C-terminus.
HIF1 heterodimers directly induce the expression of Twist by binding to HREs in the Twist proximal promoter region and promote EMT and metastatic phenotype.6 As~90% of NPC are undifferentiated (World Health Organization type III), EMT is expected to be a key feature in the oncogenesis of NPC.
Previously, we found a relation between LMP1 and increased HIF1α expression in both lymphoblastoid and epithelial cell lines.7 Subsequently, we showed the mechanism by which LMP1 functions in this content: it stabilizes seven in absentia homolog1, an E3 ubiquitin ligase, which in turn ubiquitinates and destabilizes prolyl hydroxylase proteins (PHD1 and PHD3) that, under normoxic conditions, are responsible for hydroxylation and subsequent degradation of HIF1α.8 In this manner, LMP1 expression effectively increases HIF1α levels leading to downstream transcription of angiogenic factors.
Exosomes are small vesicles (40–100 nm in diameter) originating from late endosomes and multivesicular bodies (MVBs), and they are secreted into the extracellular milieu after fusion of MVBs with the plasma membrane.9 Exosomes contain lipids, proteins and RNAs,10 and whereas some of these molecules represent obsolete cargo targeted for extracellular destruction, certain proteins and mRNAs exported by exosomes carry signaling-relevant information.11 Therefore, exosomes have recently been implicated in initiation and progression of many diseases, including cancer.12 Exosomes can transfer invasion and metastasis factors to uninfected cells and produce cellular changes characteristic of tumor progression,13 and functional delivery of LMP1 to target cells via exosomes has also been described.14 However, the mechanism of exosome formation, secretion and uptake, as well as the physiological significance of exosomal content remain to be understood.15
A large family of membrane receptors, tetraspanins, has been proposed to have major function in exosome formation and release.16 For example, expression of the tetraspanins CD9 or CD82 induces exosomal sorting and secretion of β-catenin from cells,17 and tetraspanins CD63 and CD81 have been shown to bind components of the exosomal sorting machinery.18 Tetraspanins, palmitoylated in the Golgi complex,19 form homodimers, which are subsequently transported to the cell surface, where they function as building blocks for tetraspanin-enriched microdomains.20 Late endosomes/MVBs can be triggered to fuse with the cell surface and release their intraluminal vesicles. Along with several other proteins such as Flotillin2 and HSC70, the tetraspanins CD63 and CD9 are commonly used markers for exosomes.21,22
Recent reports suggest that tumor viruses such as EBV and KSHV manipulate the tumor microenvironment through the secretion of specific viral and cellular components into exosomes.23 The exosomes produced by EBV-infected NPC cells contain high levels of LMP1 and viral microRNAs that activate critical signaling pathways in recipient cells,14 and the EBV primary oncogene LMP1 promotes secretion of pro-invasive fibroblast growth factor 2 through exosomes.24 Here, for the first time, we demonstrate that the pro-metastatic transcription factor HIF1 is abundantly secreted from NPC cells expressing LMP1 by endocytosis/exocytosis. Moreover, exosomal cell-to-cell transmission of transcriptionally active HIF1α correlated with EMT-associated changes in the cadherin expression profile in recipient cells. We propose that LMP1-positive exosomes promote progression of NPC by increasing its invasive potential and that transcription factor HIF1 participates in this process.
Results
The EBV oncogene LMP1 increases endogenous levels of the transcription factor HIF1α in exosomes secreted by NP cells
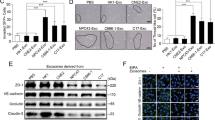
It is becoming increasingly clear that exosomes participate in distinct biological processes, including invasion and metastasis.13 As reports of the number and physiological spectrum of cellular molecules transferred intercellularly within exosomes are growing,12 we asked whether transcription factors with multifunctional effects such as HIF1α might be among important pro-invasive factors transferred from donor to recipient cells by LMP1-positive exosomes. To test this hypothesis, we analyzed exosomal fractions from both stably LMP1-expressing NP69 and LMP1 transiently transfected AdAH nasopharyngeal cells for the presence of HIF1α. To purify exosomes from the culture supernatant fluids, we used sequential centrifugation.14 Figure 1a (left panel) shows that exosomal fractions from LMP1-NP69 cells contain relatively high levels of HIF1α compared with exosomes from parental, LMP1-negative NP69 cells. Additionally, although we detected certain levels of HIF1α in exosome fractions from nasopharyngeal AdAH cells, there was a significant increase in the amount of HIF1α after transient transfection with LMP1 as illustrated in Figure 1a (right panel). Total protein lysates of pellets from the supernatant fluids were normalized for specific exosomal markers HSC70 and flotillin 2. These results indicate that exosomes from transformed NP cells contain endogenous HIF1α and that the EBV primary oncogene LMP1 upregulates amounts of the factor detected in the exosomes.
The EBV oncogene LMP1 increases endogenous levels of the transcription factor HIF1α in exosomes secreted by NP cells. (a) Purified exosomal fractions from NP69 cells stably expressing LMP1 or the control and AdAH nasopharyngeal cells transiently transfected with LMP1 or a control-expression vector were analyzed by immunoblotting. Total protein lysates of exosome pellets, isolated from supernatant fluids, were normalized to exosome-specific markers HSC70 and flotillin2. (b) Purified exosomes from NP69-LMP1 and 293T cells transiently transfected with HIF1α expression plasmids were subjected to ultrastructural examination and immunogold labeling for HIF1α. Exosomes were stained with HIF1α primary and colloidal gold-labeled secondary antibodies. (c) LMP1-negative AdAH cells were treated with graduated amounts of exosome fractions isolated from LMP1-expressing AdAH cells. After 24 h, western blotting analyses were used to detect LMP1, HIF1α and GAPDH (glyceraldehyde 3-phosphate dehydrogenase) expression in recipient AdAH cells.
We next examined the purified fractions by transmission electron microscopy (Figure 1b). Negative staining revealed vesicles 60–100 nm in diameter (Figure 1b, upper panel) with double membranes—characteristics specific for exosomes.26 Then we performed transmission electron microscopic analysis of the same exosomal nanoparticles with HIF1α primary and colloidal gold-labeled secondary antibodies (Figure 1b, bottom panel).24 HIF1α was detected inside exosomes from both the cell types examined: LMP1-NP69 and 293T cells transfected with HIF1α expression vectors. Therefore localization of HIF1α to exosomes is not exclusive to nasopharyngeal cells and is expected to be found in exosomes secreted by different types of transformed cells. In addition, we confirmed the correlation between LMP1 and HIF1α levels after exosome uptake in recipient cells: Figure 1c demonstrates that after the LMP1-negative NP cells were exposed to graduated amounts of LMP1-positive exosomes there was a dose-dependent increase of endogenous HIF1α in recipient cells as well.
Exosomal HIF1α maintains DNA-binding activity and is transcriptionally active in the recipient cells
Our previous studies demonstrate that LMP1 upregulates HIF1α expression levels in nasopharyngeal cells.7 Therefore the increase in HIF1α endogenous protein levels observed in recipient cells after treatment with LMP1-positive exosomes (Figure 1) could be caused indirectly by LMP1-mediated upregulation of HIF1α already expressed in the recipient cells. To begin establishing a direct, LMP1-independent role of HIF1α in exosome-mediated changes in recipient cells we asked: is HIF1α transferred by exosomes transcriptionally active in recipient cells?
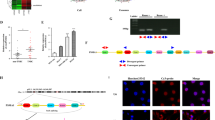
To answer this question, we utilized two HA-tagged HIF1α expression vectors; wild-type (WT) HIF1α and a deletion mutant of HIF1α that functions in a dominant-negative fashion: DN-HIF1α.27 Western blotting and luciferase reporter analyses in transiently transfected 293T (LMP1-negative) host cells show relatively equal expression of WT HIF1α and DN-HIF1α proteins and, as expected, inhibition of transcriptional activity induced by the DN HIF1α compared with WT HIF1α (Figure 2a). We next analyzed the exosome fractions from the same transfected 293T host cells for HA-tagged proteins. Figure 2b shows readily detectable levels of both WT and DN HA-HIF1α as well as exosome markers flotillin2 and HSC70. Next, we analyzed transcriptional activity of exosomal WT HIF1α and DN mutants in the recipient cells after treatment with the exosomes obtained from the cells transfected with WT and DN HIF1α. The results of the luciferase assays in Figure 2c show that HIF1α activity is markedly reduced in the recipient cells after uptake of DN HIF1α-containing exosomes. In addition, to investigate whether HIF1 presence in exosomes affects its DNA-binding ability, we performed in vitro electrophoretic mobility shift assay with exosomal HIF1 from 293T cells transfected with pcDNA3 or HA-HIF1α and oligonucleotides corresponding to HRE sequence from the Twist promoter (Figure 2d). Detection of DNA complexes with SYBR green DNA stain and western blotting analysis with HIF1α antibody showed that incubation of exosome fractions from HIF1α-transfected cells resulted in motility shift of the HRE/HIF1 complex, indicating that exosomal HIF1 specifically binds to the HRE DNA sequence. Taken together, the results in Figure 2 clearly demonstrate that the functionality of HIF1α present in exosomes is preserved.
Exosome-mediated transfer of functionally active HIF1α to recipient cells. (a) 293T cells (LMP1-negative) were transfected with pcDNA3 control and HA-tagged WT or HA-tagged DN HIF1α expression vectors along with HRE-luciferase reporter plasmid (see Materials and methods). Luciferase assays for HIF1α activity and western blotting for determination of HA-tagged proteins were performed 24 h post transfection. (b) Exosomes from the same transfected cells were purified, as described in Materials and methods. The presence of HIF1α WT and DN mutant in exosomes was determined by western blotting with HA-tag antibodies. Exosomal fractions were normalized to HSC70 and flotillin2 (exosomal markers). (c) Recipient 293T cells were transfected with HRE-luciferase reporter and exposed to purified control, WT or DN HIF1α-positive exosomes as described in panel (b). Twenty-four hours later, luciferase assays were performed and normalized to β-galactosidase activity. The mean s.ds. were determined for experiments performed in triplicate. (d) The DNA-binding capacity of exosomal HIF1α was determined by in vitro electrophoretic mobility shift assay (EMSA): exosome protein fractions were incubated with HRE probe (see Materials and methods) according to the BD manufacturer’s protocol and separated by non-denaturating 6% PAGE. To detect shifts of HRE–HIF1 complexes, the gel was stained with SYBR green EMSA to visualize DNA (left), and after transfer, the membrane was probed with HIF1α antibodies for specific identification of exosomal HIF1 in complex with the HRE promoter (top right). Coomassie staining was used for total protein levels (bottom right).
Endogenous cytoplasmic HIF1α is associated with CD63-positive late endosomes
Exosomes derive from cellular compartments collectively named ‘late endosomes’ or ‘multivesicular bodies'. The member of the tetraspanin protein family CD63 is abundant in MVBs and cycles between these compartments and the exocytic pathway.28 As a result, CD63 is one of the most reliable markers for MVBs.29 We detected endogenous HIF1α in exosomes produced by the LMP1-positive NP69 cell line (Figure 1), and because exosomes are released from the lumina of late endosomes/MVBs, we should be able to detect HIF1α in late endosomes as well. Indeed, we detected endogenous HIF1α in at least some of the late endosomal structures in NP69 cells as determined by co-localization with CD63—a marker of both exosomes and MVBs and shown by arrows in Figure 3a.
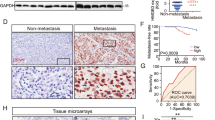
Physiological correlation between MVB marker and LMP1/HIF1α levels. (a) LMP1-positive NP69 cells were fixed in 4% paraformaldehyde and co-stained with the late endosome (MVB) marker CD63 (green) and HIF1α (red). Shown are representative images. (b) Direct correlation between MVB marker and LMP1 expression levels in NPC tissue samples was determined. Representative images of immunohistochemical analysis of paraffin-embedded NPC specimens with CD63 (top, left) and LMP1 (top, right) antibodies are presented. Dark-brown staining indicates CD63 and LMP1, respectively. In addition, expression scores for CD63 and LMP1 in 20 NPC tissue specimens (see Materials and methods) were determined. Average percentages of tumor cells staining for LMP1 and CD63 are plotted. Linear regression analyses were used to ascertain the correlation between CD63 and LMP1 expression levels (bottom).
Direct correlation between MVB marker and LMP1 expression levels in NPC tissue samples
Based on our observation that more HIF1α is present in exosomes from LMP1-positive cells (Figure 1a), and as the exosomes are formed by reverse budding of the membrane of MVB, we next analyzed possible correlation between LMP1 and MVBs levels in vivo in NPC tissues. Immunohistochemical analysis of MVB marker CD63 and LMP1 was performed in NPC specimens obtained from 20 patients (Figure 3b). The mean expression scores for CD63 and LMP1 were 21.85±24.45% and 12.05±16.17%, respectively. Expression scores for CD63 and LMP1 are closely correlated (r=0.7153; P<0.001). Taken together, our data on the association of endogenous HIF1α with MVB marker in an LMP1-positive NP cell line (Figure 3a), and the correlation between LMP1 and MVB marker in NPC tissues (Figure 3b), imply that LMP1 might upregulate exocytosis during the progression of this EBV-positive malignancy.
LMP1-positive exosomes and exosomal HIF1α change expression of EMT markers in recipient cells
LMP1 upregulates each step of metastasis and contributes to the highly metastatic character of NPC.5 Also, recent studies indicate that cancer-derived exosomes can signal activation of genes that promote cancer invasion and metastasis.13 As LMP1 induces EMT in NPC,5 we first investigated whether LMP1-positive exosomes mediate expression of EMT-associated markers in recipient cells. Adhesion molecules are of crucial importance in cancer invasion and metastasis and a major characteristic of EMT is reduction in E-cadherin and increase in N-cadherin expression.30 Western blotting analysis revealed that, after the treatment with LMP1-positive exosomes, recipient cells expressed less E-cadherin and more N-cadherin (Figure 4a), which is consistent with EMT-associated phenotype. Furthermore, Figure 4b shows that the increase in endogenous levels of N-cadherin, along with expected morphological spindle-like changes in cell shape, occurred explicitly in cells that acquired exosomes from Flag-LMP1-transfected cells, which was determined by co-staining the recipient cells with Flag and N-cadherin antibodies (Figure 4b).
LMP1-positive exosomes and exosomal HIF1α change expression of EMT markers in recipient cells. (a) LMP1-negative NP cells were exposed to purified exosomes secreted by LMP1-positive and -negative cells. Twenty-four hours later, expression levels of endogenous HIF1α, E-cadherin and N-cadherin were determined in recipient cell lysates by western blotting analyses. GAPDH (glyceraldehyde 3-phosphate dehydrogenase) levels served as loading control. (b) AdAH cells were incubated with LMP1-expressing exosomes from Flag-LMP1-transfected cells for 24 h, and then the cells were fixed and stained with Flag and N-cadherin antibodies ( × 20 (top) and × 40 (bottom) magnification, respectively). The Flag staining (red) indicates the delivery of the exosomal cargo to the recipient cells. The absence of the Flag staining served as a negative control for exosomal uptake. Anti-N-cadherin (green) staining shows the increase in N-cadherin endogenous levels in the cells that received the exosomes. (c) Recipient 293T cells (LMP1-negative) were exposed to purified exosomes secreted by cells transfected with control, WT HIF1α and DN HIF1α vectors. Exosome-mediated delivery of HIF1α proteins to recipient cells was evaluated by staining with HA-tag antibodies. Changes in endogenous E- and N-cadherin protein levels in recipient cells after exosome uptake were analyzed with the corresponding antibodies.
Next, to distinguish the role of HIF1α transcriptional activity in exosome-mediated changes in E- and N-cadherin expression levels in NP cells, we once more utilized WT and DN HIF1α. As shown in Figure 4c, exosomes with WT HIF1α from transfected 293T cells increased the levels of N-cadherin and decreased levels of E-cadherin in recipient cells, whereas an inactive deletion mutant of HIF1α from exosomes induced the opposite effect (Figure 4c, second and third lanes). Taken together, the results in Figure 4 demonstrate first that LMP1-positive exosomes induce EMT-associated marker in recipient NP cells and second that active HIF1α is likely to be a part of such exosome-mediated changes.
LMP1-positive exosomes increase migration and invasiveness of NP cells in functional motility assays
To determine whether the upregulation of EMT-associated markers by LMP1-positive exosomes has physiological consequences, we utilized two cell culture assays commonly used to analyze cell motility and invasiveness: the scratch (wound/healing) assay and the migration of cells in extracellular matrix (Matrigel) assay. Figure 5a demonstrates that migration of both LMP1-negative NP69 and AdAH cell lines on the surface after scratching was significantly increased by exposure to the LMP1-exosomes. Moreover, we obtained concordant results in the trans-migration assay as well. LMP1-exosome uptake stimulated trans-migration of LMP1-negative NP cells into Matrigel (Figure 5b). Although exosome treatment is likely to change many cellular processes such as cell cycle progression, we found fewer exosome-treated cells after 24 h in the upper chamber compared with the control, untreated cells. Hence, our findings suggest that the cargo molecules, such as HIF1 in the LMP1-positive exosomes, specifically change cell motility. Taken together, these results support our hypothesis that exosomes secreted by LMP1-positive cells participate in pro-metastatic progression of EBV-positive NPC, presumably through the induction of EMT.
LMP1-positive exosomes increase motility and invasiveness of NP cells in functional assays. (a) Sub-confluent LMP1-negative AdAH and NP69 cells that had been incubated with or without LMP1-expressing exosomes were scratched and cultured for 24 h. The widths of the ‘wound’ (scratched areas) were measured by ImageJ software (http://rsb.info.nih.gov/ij/), and the percentage of the wound healed was calculated by the following formula: ‘wounded area filled (%)’=100%−(width after 24 h/width at beginning) × 100% as shown in the histogram. At left is a representative image of AdAH control and LMP1-exosome-treated cells 0 and 24 h after the scratch was applied (*P<0.01, T-test). (b) The suspension of NP69 and AdAH cells incubated with or without LMP1-expressing exosomes was added to the insert of a transwell cell-culture chamber containing Matrigel and incubated for 24 h. Medium containing 10% fetal bovine serum was added to the bottom of the chamber. Cells that attached to the underside of the membrane were fixed and counted, and the average number of cells per field of view was determined (mean±s.d.; n=3; *P<0.01, T-test). At left, representative images of control and LMP1-exosome-treated AdAH cells that invaded into the Matrigel after 24 h, stained with crystal violet.
Discussion
The main function of exosomes is intercellular communication, which can result in redirection of signaling pathways in recipient cells after exposure to the exosomes.11 Previous studies show that the viral oncogene LMP1 as well as EBV microRNAs can be transmitted from cell to cell by exosomes,31 and such transmission is associated with EBV-induced oncogenesis.32
Reports of the variety of biologically active molecules that can be transported intercellularly by exosomes are increasing.11 Indeed, exosomes secreted by virally infected cells contain >100 biologically active molecules.23 Although viral DNA and microRNAs have been consistently detected in the peripheral blood of NPC patients, the first evidence physiologically connecting EBV-positive NPCs to exosomes came from observations showing that exosomal fractions from the saliva, serum and plasma of NPC patients contain EBV LMP1 and BARF1 oncoproteins.32 Further studies demonstrated that LMP1-positive exosomes from latently EBV-infected cells can transfer many signaling molecules to the recipient cells.33 However, the present study is the first to reveal and analyze the presence of a key pro-metastatic transcriptional factor HIF1α in LMP1-exosomes (Figure 1). Most importantly, our observations demonstrate biological activity of this transcriptional factor after exosomal delivery to the recipient cells (Figure 2).
There is indirect evidence of involvement of HIF1α in exosomal pathways: exosomes from glioma patients are enriched in hypoxia-regulated mRNAs and proteins.34 The secretion of exosomes under hypoxic conditions within the placenta is associated with proliferation and invasion of extravillous trophoblasts.35 In addition, HIF1α initiates tumor necrosis factor-α exosome-mediated secretion under hypoxic conditions.36 The localization of HIF1α to exosomes does not depend on the viral oncogene, because it is detectable in exosomal fractions of LMP1-negative cells as well, but the presence of LMP1 significantly increases the amount of HIF1α in these extracellular structures (Figure 1).
As the intracellular sources of exosomes are MVBs, we also demonstrate the presence of endogenous HIF1α in these cellular structures in NP cells (Figure 3a). The findings indicate a specific mechanism for sorting HIF1α to MVBs, distinct from targeting for destruction by lysosomes. So far, several hypotheses have been proposed for MVB-mediated mechanism of exosomal cargo formation,37, 38, 39 but the precise mechanism remains to be determined. It has been shown recently that regulatory ubiquitination is required for export of PTEN tumor suppressor to exosomes.40 A similar mechanism might be involved in exosomal intake of HIF1α as well. In addition, a possible role for LMP1 in exosome biogenesis cannot be eliminated. Our analyses also revealed the correlation between LMP1 and CD63 levels in NPC tissues (Figure 3b). In the future, we plan to investigate specific mechanisms of exosome formation and cargo transfer in metastatic LMP1-positive NPC. Taken together, our data on the association of endogenous HIF1α with MVB marker in an NP cell line (Figure 3a), and the correlation between LMP1 and MVB marker in NPC tissues (Figure 3b), imply that LMP1 might regulate the processing of exosomes during NPC metastatic progression.
In this study, we show that LMP1-positive exosomes (Figures 4a and b) and HIF1α specifically (Figure 4c) can counterbalance the levels of E- and N-cadherin in the recipient cells, consistent with EMT-associated changes. More importantly, we demonstrate in two different assays that exposure of non-malignant NP cells to LMP1-positive exosomes with high levels of pro-metastatic factor HIF1α increases the motility of the NP cells and thus enhances their invasive potential (Figure 5). Recent reports indicate that tumor-secreted exosomes manipulate the metastatic cascade through angiogenesis, stromal remodeling and signal transduction by growth factor/receptor transfer, chemoresistance and genetic intercellular exchange.41 HIF1 is likely not the only transcription factor involved in these metastatic exosome-mediated effects (although so far we did not detect in exosomal fractions other possible candidates, such as Twist or Snail transcription factors).
From the perspective of the other physiological roles of LMP1-exosomes in the malignant properties of oncogenesis of NPC, we also suggest that the cargo of the LMP1-positive exosomes (including HIF1) transmits pro-metastatic signals to surrounding cells as well. Malignant tumors are a complex of histopathological features that consist of cancer cells and stroma.42 It is likely that exosome-mediated transfer of pro-metastatic factors is one of the major ways for highly invasive EBV-positive NPC cells to induce EMT-like changes in tissues surrounding tumors. Continuous crosstalk between cancer cells and local as well as distant host environment is required for aggressive tumor growth and systemic dissemination.43
In summary, rapidly emerging evidence indicates that exosomes may well have pivotal roles in local and systemic cell–cell communication in the progression of nasopharyngeal cancer.32 Thus the major implication of the recent data is that LMP1-exosomes affect NPC stromal components as well as tumor cells themselves during invasion and metastasis. Pro-metastatic transcription factors in exosomes such as HIF1α secure immediate changes in the expression of sets of genes in recipient cells. Hence, HIF1α-containing LMP1-exosomes offer not only new opportunities for biomarker analysis but may also represent targets for therapeutic intervention in metastatic NPC.
Materials and methods
Cell culture
Ad-AH cells, an EBV-negative human NPC (NP) cell line, were kindly provided by Dr Erik K Flemington (Tulane University, New Orleans, LA, USA) and maintained in DMEM (Dulbecco’s modified Eagle’s medium). NP69 and NP69LMP1, human immortalized NP epithelial cells, generous gifts from Dr Sai Wai Tsao (University of Hong Kong, Hong Kong, China), were maintained in keratinocyte serum-free medium. All cells were maintained at 37 °C in 5% CO2. Human embryonic kidney 293T cells were maintained in DMEM.
Antibodies
Antibodies were purchased as indicated: HIF1α, N-cadherin, GAPDH, CD63, HA and anti-HSC70 (from Santa Cruz Biotechnology, Santa Cruz, CA, USA); and E-cadherin and anti–flotillin-2 (from BD Biosciences, San Jose, CA, USA). S12 anti-LMP1 antibody was obtained from supernatant fluid of a hybridoma produced by and the generous gift of David A Thorley-Lawson (Tufts University).
Plasmids
pcDNA3-based LMP1 has been previously described.7 HA-HIF1α and HRE reporter plasmid were purchased from Addgene (Cambridge, MA, USA) (Plasmid 26731). Luciferase reporter construct containing three HREs (HRE: TGTCACGTCCTGCACGACTCTAGT) was from the Pgk-1 gene. HA-DNHIF1α was a gift from Darren E Richard.
Immunofluorescence microscopy
Cells cultured on glass coverslips were fixed with 4% paraformaldehyde, washed with phosphate-buffered saline (PBS), permeabilized with 0.5% Triton X-100/PBS and blocked with 1% normal donkey serum. Cells were then incubated with primary antibodies overnight and were the same as used for western blotting.
Western blotting analysis
Total cell lysates were denatured in sodium dodecyl sulfate (SDS) (Sigma, St Louis, MO, USA) loading buffer and boiled for 5 min. Samples were separated by SDS-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to polyvinylidene fluoride (PVDF) membranes (General Electric, Fairfield, CT, USA). Membranes were blocked with 5% milk in Tris-buffered saline–Tween 20 (TBST) and incubated overnight at 4 °C with primary antibodies. Membranes were then washed and incubated with appropriate horseradish peroxidase-conjugated secondary antibodies for 1 h at room temperature. Membranes were washed again, and bands were visualized with enhanced chemiluminescence reagent from GE.
Transient transfection and Luciferase assays
293T cells were grown in six-well plates and transfected with 1 μg of plasmids with the use of polyethylenimine (VWR, Radnor, PA, USA). Empty vector was used to equalize total amounts of DNA in the transfections. β-Galactosidase served as a transfection control. Luciferase and β-galactosidase assays were performed 24 h posttransfection.
Exosome isolation and transfer
293T cells were grown to confluent monolayers and grown in culture for an additional day. Exosomes were collected by differential centrifugation from conditioned media, resuspended into PBS and stored at−80 °C until use.25 Cell monolayers were washed twice with PBS and incubated with purified exosomes for the indicated times at 37 °C in serum-free media. Cells exposed to exosomes were washed three times with PBS, scraped into cold PBS, pelleted and lysed in PBS. HRE-luciferase plasmid and β-galactosidase plasmid were transfected into 293T cells before exposure to exosomes.
Invasiveness and wound-healing assays
For invasiveness assays, Matrigel was purchased from BD Biosciences and stored at−20 °C. Matrigel invasiveness assays were carried out according to the manufacturer's instructions. The cell suspension with or without exosomes from LMP1-expressing cells was added to the upper chamber of a six-well transwell cell culture chamber and was incubated for 24 h with medium containing 10% fetal bovine serum in the bottom of the chamber. Cells adherent to the lower surface of filters were fixed and stained, and cells invading through the Matrigel-coated membrane were counted. For wound-healing assays, confluent cell monolayers incubated with or without exosomes from LMP1-expressing cells were scratched with a micropipette tip, and spontaneous cell migration was monitored for 24 h. The widths of the ‘wound’ (scratched areas) were measured by ImageJ (http://rsbweb.nih.gov/ij/), and the proportion of wound healing was calculated by the following formula: 100%−(width after 24 h/width at the beginning) × 100%.
Immunoelectron microscopy
Exosome-containing pellets obtained by ultracentrifugation were fixed with 4% paraformaldehyde in PBS for 2 h at room temperature and then resuspended in 2% paraformaldehyde in PBS. Droplets of this exosomal fraction were directly spotted onto Formvar/carbon-coated copper grids (100 meshes) and incubated with anti-HIF1α monoclonal antibody. After several rinses in PBS-0.1% bovine serum albumin, the grids were incubated with 18-nm diameter colloidal gold particles (prepared by citrate method) conjugated with protein A (Pharmacia, Uppsala, Sweden) and diluted in 1% bovine serum albumin in PBS. Control experiments were performed by omission of the primary antibodies from the labeling procedure. Finally, grids were stained with a solution of 2% methyl cellulose and 0.4% uranyl acetate before examination by transmission electron microscopy.24
Electrophoretic mobility shift assay
Mobility shift assays (Invitrogen, Carlsbad, CA, USA, E-33075) were performed with the following double-stranded oligonucleotides containing HIF1 binding site (HRE) surrounding sequences from the human twist promoter:
5′-GCGAGTCTCAGCAGGGAACAGCCACGTGGCCTGCCTGCGCCTCGCCTGGGCT-3′ and 5′-AGCCCAGGCGAGGCGCAGGCAGGCCACGTGGCTGTTCCCTGCTGAGACTCGC-3′. This oligonucleotide was generated using single-stranded oligonucleotide. The oligomers (3 mg/ml) were annealed to complementary oligomer in 3 mM sodium citrate, pH 7.0 and 30 mM NaCl at 95 °C for 5 min and then cooled slowly to room temperature for several hours. The annealed oligomers were incubated with exosomes from 293T cells transfected with pcDNA3 or HA-HIF1α in the presence of 5 × binding buffer (Invitrogen; E33075) for 20 min at room temperature. At the end of the reaction, DNA loading dye was added, and the reaction was separated on 6% PAGE in TAE buffer at 200 V. The gel was then stained with SYBR green DNA stain for 20 min, washed with double-distilled H2O and visualized with a fluorescence imager (Kodak, Rochester, NY, USA). Next, for detecting HIF1α, proteins were transferred onto PVDF membrane, and western blotting analysis was performed for HIF1α with anti-HIF1α antibody.
Immunohistochemical analysis of NPC tissues
Twenty selected NPC paraffin-embedded specimens from Kanazawa University Hospital (Ishikawa, Japan) and Toyama Prefectural Central Hospital (Toyama, Japan) were used for detection of LMP1 and CD63. Specimens were from 18 men and 2 women, aged 43–78 years (mean 58.5 years) and classified histologically as follows: 1 keratinizing carcinoma, 12 nonkeratinizing carcinoma (type II), and 7 undifferentiated carcinoma (type III) (Fifth TNM classification system of the International Union Against Cancer (1997)). Primary antibody for CD63 was the same as used for western blotting. Mouse LMP1 antibody was from DAKO (Glostrup, Denmark). Stained samples of CD63 and LMP1 were evaluated as follows: under light microscopy, two areas that showed high densities of stained cells were selected in a × 40 field. The number of stained cells among 200 cells was then carefully counted in two areas in a × 200 field without knowledge of clinicopathological status. Percentages of positive cells obtained as an average of two counts were used as expression scores for both proteins.
References
Daker M, Ahmad M, Khoo AS . Quercetin-induced inhibition and synergistic activity with cisplatin - a chemotherapeutic strategy for nasopharyngeal carcinoma cells. Cancer Cell Int 2012; 12: 34.
Raab-Traub N . Novel mechanisms of EBV-induced oncogenesis. Curr Opin Virol 2012; 2: 453–458.
Yoshizaki T, Wakisaka N, Pagano JS . Epstein-Barr virus invasion and metastasis. In: Robertson Erle S (ed).Epstein-Barr Virus, Caister Academic Press: Philadelphia, PA, USA, 2005, pp171–196.
Thiery JP, Sleeman JP . Complex networks orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell Biol 2006; 7: 131–142.
Yoshizaki T, Kondo S, Wakisaka N, Murono S, Endo K, Sugimoto H et al. Pathogenic role of Epstein-Barr virus latent membrane protein-1 in the development of nasopharyngeal carcinoma. Cancer Lett 2013; 337: 1–7.
Yang MH, Wu KJ . TWIST activation by hypoxia inducible factor-1 (HIF1): implications in metastasis and development. Cell Cycle 2008; 7: 2090–2096.
Wakisaka N, Kondo S, Yoshizaki T, Murono S, Furukawa M, Pagano JS . Epstein-Barr virus latent membrane protein 1 induces synthesis of hypoxia-inducible factor 1 alpha. Mol Cell Biol 2004; 24: 5223–5234.
Kondo S, Seo SY, Yoshizaki T, Wakisaka N, Furukawa M, Joab I et al. EBV latent membrane protein 1 up-regulates hypoxia-inducible factor 1alpha through Siah1-mediated down-regulation of prolyl hydroxylases 1 and 3 in nasopharyngeal epithelial cells. Cancer Res 2006; 66: 9870–9877.
Johnstone RM . Exosomes biological significance: a concise review. Blood Cells Mol Dis 2006; 36: 315–321.
Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ, Lötvall JO . Exosomemediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol 2007; 9: 654–659.
Denzer K, Kleijmeer MJ, Heijnen HF, Stoorvogel W, Geuze HJ . Exosome: from internal vesicle of the multivesicular body to intercellular signaling device. J Cell Sci 2000; 113: 3365–3374.
Peinado H, Alečković M, Lavotshkin S, Matei I, Costa-Silva B, Moreno-Bueno G et al. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat Med 2012; 18: 883–891.
Hong BS, Cho JH, Kim H, Choi EJ, Rho S, Kim J et al. Colorectal cancer cell-derived microvesicles are enriched in cell cycle-related mRNAs that promote proliferation of endothelial cells. BMC Genomics 2009; 10: 556.
Meckes DG Jr, Shair KH, Marquitz AR, Kung CP, Edwards RH, Raab-Traub N . Human tumor virus utilizes exosomes for intercellular communication. Proc Natl Acad Sci USA 2010; 107: 20370–20375.
Gourzones C, Barjon C, Busson P . Host-tumor interactions in nasopharyngeal carcinomas. Semin Cancer Biol 2012; 22: 127–136.
Rana S, Zöller M . Exosome target cell selection and the importance of exosomal tetraspanins: a hypothesis. Biochem Soc Trans 2011; 39: 559–562.
Chairoungdua A, Smith DL, Pochard P, Hull M, Caplan MJ . Exosome release of β-catenin: a novel mechanism that antagonizes Wnt signaling. J Cell Biol 2010; 190: 1079–1091.
Escola JM, Kleijmeer MJ, Stoorvogel W, Griffith JM, Yoshie O, Geuze HJ . Selective enrichment of tetraspan proteins on the internal vesicles of multivesicular endosomes and on exosomes secreted by human B-lymphocytes. J Biol Chem 1998; 273: 20121–20127.
Yang X, Claas C, Kraeft SK, Chen LB, Wang Z, Kreidberg JA et al. Palmitoylation of tetraspanin proteins: modulation of CD151 lateral interactions, subcellular distribution, and integrin-dependent cell morphology. Mol Biol Cell 2002; 13: 767–781.
Kovalenko OV, Yang X, Kolesnikova TV, Hemler ME . Evidence for specific tetraspanin homodimers: inhibition of palmitoylation makes cysteine residues available for cross-linking. Biochem J 2004; 377: 407–417.
Mathivanan S, Ji H, Simpson RJ . Exosomes: extracellular organelles important in intercellular communication. J Proteomics 2010; 73: 1907–1920.
Simpson RJ, Lim JW, Moritz RL, Mathivanan S . Exosomes: proteomic insights and diagnostic potential. Expert Rev Proteomics 2009; 6: 267–283.
Meckes DG Jr, Gunawardena HP, Dekroon RM, Heaton PR, Edwards RH, Ozgur S et al. Modulation of B-cell exosome proteins by gamma herpesvirus infection. Proc Natl Acad Sci USA, in press110: E2925–E2933.
Ceccarelli S, Visco V, Raffa S, Wakisaka N, Pagano JS, Torrisi MR . Epstein-Barr virus latent membrane protein 1 promotes concentration in multivesicular bodies of fibroblast growth factor 2 and its release through exosomes. Int J Cancer 2007; 121: 1494–1506.
Staubach S, Razawi H, Hanisch FG . Proteomics of MUC1-containing lipid rafts from plasma membranes and exosomes of human breast carcinoma cells MCF-7. Proteomics 2009; 9: 2820–2835.
Heijnen HF, Schiel AE, Fijnheer R, Geuze HJ, Sixma JJ . Activated platelets release two types of membrane vesicles: microvesicles by surface shedding and exosomes derived from exocytosis of multivesicular bodies and alpha-granules. Blood 1999; 94: 3791–3799.
Richard DE, Berra E, Pouyssegur J . Nonhypoxic pathway mediates the induction of hypoxia-inducible factor 1alpha in vascular smooth muscle cells. J Biol Chem 2000; 275: 26765–26771.
Kobayashi T, Vischer UM, Rosnoblet C, Lebrand C, Lindsay M, Parton RG et al. The tetraspanin CD63/lamp3 cycles between endocytic and secretory compartments in human endothelial cells. Mol Biol Cell 2000; 11: 1829–1843.
Lamparski HG, Metha-Damani A, Yao JY, Patel S, Hsu DH, Ruegg C et al. Production and characterization of clinical grade exosomes derived from dendritic cells. J Immunol Methods 2002; 270: 211–226.
Le Bras GF, Taubenslag KJ, Andl CD . The regulation of cell-cell adhesion during epithelial-mesenchymal transition, motility and tumor progression. Cell Adh Migr 2012; 6: 365–373.
Pegtel DM, Cosmopoulos K, Thorley-Lawson DA, van Eijndhoven MA, Hopmans ES, Lindenberg JL et al. Functional delivery of viral miRNAs via exosomes. Proc Natl Acad Sci USA 2010; 107: 6328–6333.
Houali K, Wang X, Shimizu Y, Djennaoui D, Nicholls J, Fiorini S et al. A new diagnostic marker for secreted Epstein-Barr virus encoded LMP1 and BARF1 oncoproteins in the serum and saliva of patients with nasopharyngeal carcinoma. Clin Cancer Res 2007; 13: 4993–5000.
Meckes DG Jr, Raab-Traub N . Microvesicles and viral infection. J Virol 2011; 85: 12844–12854.
Kucharzewska P, Christianson HC, Welch JE, Svensson KJ, Fredlund E, Ringnér M et al. Exosomes reflect the hypoxic status of glioma cells and mediate hypoxia-dependent activation of vascular cells during tumor development. Proc Natl Acad Sci USA 2013; 110: 7312–7317.
Salomon C, Kobayashi M, Ashman K, Sobrevia L, Mitchell MD, Rice GE . Hypoxia-induced changes in the bioactivity of cytotrophoblast-derived exosomes. PLoS One 2013; 8: e79636.
Yu X, Deng L, Wang D, Li N, Chen X, Cheng X et al. Mechanism of TNF-α autocrine effects in hypoxic cardiomyocytes: initiated by hypoxia inducible factor 1α, presented by exosomes. J Mol Cell Cardiol 2012; 53: 848–857.
Corrado C, Raimondo S, Chiesi A, Ciccia F, De Leo G, Alessandro R . Exosomes as intercellular signaling organelles involved in health and disease: basic science and clinical applications. Int J Mol Sci 2013; 14: 5338–5366.
Pant S, Hilton H, Burczynski ME . The multifaceted exosome: biogenesis, role in normal and aberrant cellular function, and frontiers for pharmacological and biomarker opportunities. Biochem Pharmacol 2012; 83: 1484–1494.
Vlassov AV, Magdaleno S, Setterquist R, Conrad R . Exosomes: current knowledge of their composition, biological functions, and diagnostic and therapeutic potentials. Biochim Biophys Acta 2012; 1820: 940–948.
Putz U, Howitt J, Doan A, Goh CP, Low LH, Silke J et al. The tumor suppressor PTEN is exported in exosomes and has phosphatase activity in recipient cells. Sci Signal 2012; 5: ra70.
Hendrix A, Hume AN . Exosome signaling in mammary gland development and cancer. Int J Dev Biol 2011; 55: 879–887.
Sund M, Kalluri R . Tumor stroma derived biomarkers in cancer. Cancer Metastasis Rev 2009; 28: 177–183.
Kalluri R, Zeisberg M . Fibroblasts in cancer. Nat Rev Cancer 2006; 6: 392–401.
Acknowledgements
We thank Meckes DG Jr. and Raab-Traub N (University of North Carolina, Chapel Hill, NC, USA) for providing helpful advice on the further experimental strategies and Richard DE (Centre de Recherche en Cancérologie de l'Université Laval, Québec, QC, Canada) for the HA-DN HIF1α plasmid. This work was supported by a grant from the National Cancer Institute 2P01-CA19014-26 and International Training Program of Cancer Research Institute of Kanazawa University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/
About this article
Cite this article
Aga, M., Bentz, G., Raffa, S. et al. Exosomal HIF1α supports invasive potential of nasopharyngeal carcinoma-associated LMP1-positive exosomes. Oncogene 33, 4613–4622 (2014). https://doi.org/10.1038/onc.2014.66
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2014.66
This article is cited by
-
Protein cargo in extracellular vesicles as the key mediator in the progression of cancer
Cell Communication and Signaling (2024)
-
Tumor-derived small extracellular vesicles in cancer invasion and metastasis: molecular mechanisms, and clinical significance
Molecular Cancer (2024)
-
Diagnostic and therapeutic value of EVs in lungs diseases and inflammation
Molecular Biology Reports (2024)
-
Engineered small extracellular vesicles as a novel platform to suppress human oncovirus-associated cancers
Infectious Agents and Cancer (2023)
-
Precision medicine in nasopharyngeal carcinoma: comprehensive review of past, present, and future prospect
Journal of Translational Medicine (2023)