Abstract
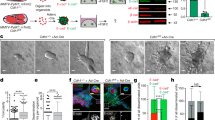
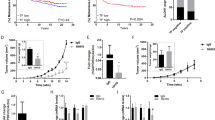
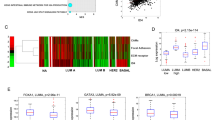
Profilin1 (Pfn1), a ubiquitously expressed actin-binding protein, has an indispensable role in migration and proliferation of normal cells. Seemingly contrary to its essential cellular functions, Pfn1’s expression is downregulated in breast cancer, the significance of which is unclear. In this study, expression profiling of Pfn1 in human breast cancer specimens correlates lower Pfn1 expression levels with propensity to metastasize. Xenograft experiments further establish a causal relationship between loss of Pfn1 expression and increased dissemination of breast cancer cells (BCCs) from the primary mammary tumor. BCCs exhibit a hyperinvasive phenotype (marked by matrix metalloproteinase-9 upregulation, faster invasion through collagen matrix) and acquire increased proficiency to transmigrate through endothelial barrier (an obligatory step for vascular dissemination) when Pfn1 expression is suppressed. In Pfn1-deficient cells, hyperinvasiveness involves a phosphatidylinositol 3-kinase-PI(3,4)P2 signaling axis while augmented transendothelial migration occurs in a vascular endothelial growth factor-dependent manner. Contrasting these dissemination promoting activities, loss of Pfn1, however, dramatically inhibits metastatic outgrowth of disseminated BCCs, suggesting that Pfn1 has a key role in the metastatic colonization process. In summary, this study shows that Pfn1 has a dichotomous role in early vs late steps of breast cancer metastasis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Gronborg M, Kristiansen TZ, Iwahori A, Chang R, Reddy R, Sato N et al. Biomarker discovery from pancreatic cancer secretome using a differential proteomic approach. Mol Cell Proteomics 2006; 5: 157–171.
Janke J, Schluter K, Jandrig B, Theile M, Kolble K, Arnold W et al. Suppression of tumorigeniciy in breast cancer cells by the microfilament protein profilin 1. J Exp Med 2000; 191: 1675–1685.
Wu N, Zhang W, Yang Y, Liang YL, Wang LY, Jin JW et al. Profilin 1 obtained by proteomic analysis in all-trans retinoic acid-treated hepatocarcinoma cell lines is involved in inhibition of cell proliferation and migration. Proteomics 2006; 6: 6095–6106.
Zoidakis J, Makridakis M, Zerefos PG, Bitsika V, Esteban S, Frantzi M et al. Profilin 1 is a potential biomarker for bladder cancer aggressiveness. Mol Cell Proteomics 2012; 11: M111 009449.
Jockusch BM, Murk K, Rothkegel M . The profile of profilins. Rev Physiol Biochem Pharmacol 2007; 159: 131–149.
Karlsson R, Lindberg U . Profilin, an essential control element for actin polymerization. In: Lapplainen P (ed). Actin Monomer Binding Proteins. Landes Biosciences and Springer,, Georgetown, USA 2006; Chapter 3: 29–44.
Witke W . The role of profilin complexes in cell motility and other cellular processes. Trends Cell Biol 2004; 14: 461–469.
Bottcher RT, Wiesner S, Braun A, Wimmer R, Berna A, Elad N et al. Profilin 1 is required for abscission during late cytokinesis of chondrocytes. EMBO J 2009; 28: 1157–1169.
Ding Z, Gau D, Deasy B, Wells A, Roy P . Both actin and polyproline interactions of profilin-1 are required for migration, invasion and capillary morphogenesis of vascular endothelial cells. Exp Cell Res 2009; 315: 2963–2973.
Ding Z, Lambrechts A, Parepally M, Roy P . Silencing profilin-1 inhibits endothelial cell proliferation, migration and cord morphogenesis. J Cell Sci 2006; 119 (Pt 19): 4127–4137.
Haugwitz M, Noegel AA, Karakesisoglou J, Schleicher M . Dictyostelium amoebae that lack G-actin-sequestering profilins show defects in F-actin content, cytokinesis, and development. Cell 1994; 79: 303–314.
Khadka DK, Liu W, Habas R . Non-redundant roles for Profilin2 and Profilin1 during vertebrate gastrulation. Dev Biol 2009; 332: 396–406.
Kullmann JA, Neumeyer A, Gurniak CB, Friauf E, Witke W, Rust MB . Profilin1 is required for glial cell adhesion and radial migration of cerebellar granule neurons. EMBO Rep 2011; 13: 75–82.
Sato A, Khadka DK, Liu W, Bharti R, Runnels LW, Dawid IB et al. Profilin is an effector for Daam1 in non-canonical Wnt signaling and is required for vertebrate gastrulation. Development 2006; 133: 4219–4231.
Severson AF, Baillie DL, Bowerman BA . Formin homology protein and a profilin are required for cytokinesis and Arp2/3-independent assembly of cortical microfilaments in C. elegans. Curr Biol 2002; 12: 2066–2075.
Verheyen EM, Cooley L . Profilin mutations disrupt multiple actin-dependent processes during drosophila development. Development 1994; 120: 717–728.
Bae YH, Ding Z, Das T, Wells A, Gertler F, Roy P . Profilin1 regulates PI(3,4)P2 and lamellipodin accumulation at the leading edge thus influencing motility of MDA-MB-231 cells. Proc Natl Acad Sci USA 2010; 107: 21547–21552.
Bae YH, Ding Z, Zou L, Wells A, Gertler F, Roy P . Loss of profilin-1 expression enhances breast cancer cell motility by Ena/VASP proteins. J Cell Physiol 2009; 219: 354–364.
Poincloux R, Collin O, Lizarraga F, Romao M, Debray M, Piel M et al. Contractility of the cell rear drives invasion of breast tumor cells in 3D Matrigel. Proc Natl Acad Sci USA 2011; 108: 1943–1948.
Zou L, Jaramillo M, Whaley D, Wells A, Panchapakesa V, Das T et al. Profilin-1 is a negative regulator of mammary carcinoma aggressiveness. Br J Cancer 2007; 97: 1361–1371.
Ho MY, Tang SJ, Chuang MJ, Cha TL, Li JY, Sun GH et al. TNF-alpha induces epithelial-mesenchymal transition of renal cell carcinoma cells via a GSK3beta-dependent mechanism. Mol Cancer Res 2012; 10: 1109–1119.
Krause M, Leslie JD, Stewart M, Lafuente EM, Valderrama F, Jagannathan R et al. Lamellipodin, an Ena/VASP ligand, is implicated in the regulation of lamellipodial dynamics. Dev Cell 2004; 7: 571–583.
Smith K, Humphreys D, Hume PJ, Koronakis V . Enteropathogenic Escherichia coli recruits the cellular inositol phosphatase SHIP2 to regulate actin-pedestal formation. Cell Host Microbe 2010; 7: 13–24.
Fedele CG, Ooms LM, Ho M, Vieusseux J, O'Toole SA, Millar EK et al. Inositol polyphosphate 4-phosphatase II regulates PI3K/Akt signaling and is lost in human basal-like breast cancers. Proc Natl Acad Sci USA 2010; 107: 22231–22236.
Gewinner C, Wang ZC, Richardson A, Teruya-Feldstein J, Etemadmoghadam D, Bowtell D et al. Evidence that inositol polyphosphate 4-phosphatase type II is a tumor suppressor that inhibits PI3K signaling. Cancer Cell 2009; 16: 115–125.
Gorges TM, Tinhofer I, Drosch M, Rose L, Zollner TM, Krahn T et al. Circulating tumour cells escape from EpCAM-based detection due to epithelial-to-mesenchymal transition. BMC Cancer 2012; 12: 178.
Chao Y, Wu Q, Acquafondata M, Dhir R, Wells A . Partial mesenchymal to epithelial reverting transition in breast and prostate cancer metastases. Cancer Microenviron 2012; 5: 19–28.
Chao YL, Shepard CR, Wells A . Breast carcinoma cells re-express E-cadherin during mesenchymal to epithelial reverting transition. Mol Cancer 2010; 9: 179.
Goldschmidt-Clermont PJ, Kim JW, Machesky LM, Rhee SG, Pollard TD . Regulation of phospholipase C-gamma 1 by profilin and tyrosine phosphorylation. Science 1991; 251: 1231–1233.
Goldschmidt-Clermont PJ, Machesky LM, Baldassare JJ, Pollard TD . The actin-binding protein profilin binds to PIP2 and inhibits its hydrolysis by phospholipase C. Science 1990; 247: 1575–1578.
Lederer M, Jockusch BM, Rothkegel M . Profilin regulates the activity of p42POP, a novel Myb-related transcription factor. J Cell Sci 2005; 118 (Pt 2): 331–341.
Barkan D, Kleinman H, Simmons JL, Asmussen H, Kamaraju AK, Hoenorhoff MJ et al. Inhibition of metastatic outgrowth from single dormant tumor cells by targeting the cytoskeleton. Cancer Res 2008; 68: 6241–6250.
Shibue T, Weinberg RA . Integrin beta1-focal adhesion kinase signaling directs the proliferation of metastatic cancer cells disseminated in the lungs. Proc Natl Acad Sci USA 2009; 106: 10290–10295.
Roy P, Jacobson K . Overexpression of profilin reduces the migration of invasive breast cancer cells. Cell Motil Cytoskeleton 2004; 57: 84–95.
Gau D, Ding Z, Baty C, Roy P . Fluorescence resonance energy transfer (FRET)-based detection of profilin-VASP interaction. Cell Mol Bioeng 2011; 4: 1–8.
Zou L, Hazan R, Roy P . Profilin-1 overexpression restores adherens junctions in MDA-MB-231 breast cancer cells in R-cadherin-dependent manner. Cell Motil Cytoskeleton 2009; 66: 1048–1056.
Kim A, Lakshman N, Karamichos D, Petroll WM . Growth factor regulation of corneal keratocyte differentiation and migration in compressed collagen matrices. Invest Ophthalmol Vis Sci 2010; 51: 864–875.
Artym VV, Yamada KM, Mueller SC . ECM degradation assays for analyzing local cell invasion. Methods Mol Biol 2009; 522: 211–219.
Acknowledgements
This work was supported by grants from the National Cancer Institute of the National Institute of Health (2R01CA108607-07) and the Magee Women’s Research Institute to PR.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Rights and permissions
About this article
Cite this article
Ding, Z., Joy, M., Bhargava, R. et al. Profilin-1 downregulation has contrasting effects on early vs late steps of breast cancer metastasis. Oncogene 33, 2065–2074 (2014). https://doi.org/10.1038/onc.2013.166
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2013.166
Keywords
This article is cited by
-
Profilin 1 deficiency drives mitotic defects and reduces genome stability
Communications Biology (2023)
-
Association of PFN1 Gene Polymorphisms with Bone Mineral Density, Bone Turnover Markers, and Osteoporotic Fractures in Chinese Population
Calcified Tissue International (2023)
-
Increased expression of Profilin potentiates chemotherapeutic agent-mediated tumour regression
British Journal of Cancer (2022)
-
Adaptor SH3BGRL promotes breast cancer metastasis through PFN1 degradation by translational STUB1 upregulation
Oncogene (2021)
-
E3 ubiquitin ligase CHIP attenuates cellular proliferation and invasion abilities in triple-negative breast cancer cells
Clinical and Experimental Medicine (2020)