Abstract
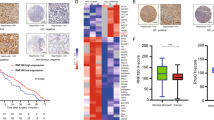
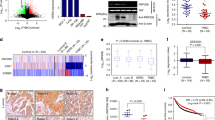
The WW domain containing E3 ubiquitin protein ligase 1 (WWP1) is a homologous to the E6-associated protein C terminus-type E3 ligase frequently overexpressed in human prostate and breast cancers due to gene amplification. Previous studies suggest that WWP1 promotes cell proliferation and survival; however, the mechanism of WWP1 action is still poorly understood. Here, we showed that WWP1 upregulates and maintains erythroblastic leukemia viral oncogene homolog 2 (ErbB2) and epithelial growth factor receptor (EGFR) in multiple cell lines. WWP1 depletion dramatically attenuates the EGF-induced ERK phosphorylation. WWP1 forms a protein complex with RING finger protein 11 (RNF11), a negative regulator of ErbB2 and EGFR. The protein–protein interaction is through the first and third WW domains of WWP1 and the PY motif of RNF11. Although WWP1 is able to ubiquitinate RNF11 in vitro and in vivo, WWP1 neither targets RNF11 for degradation nor changes RNF11's cellular localization. Importantly, inhibition of RNF11 can rescue WWP1 siRNA-induced ErbB2 and EGFR downregulation and growth arrest. Finally, we demonstrated that RNF11 is overexpressed in a panel of prostate and breast cancer cell lines with WWP1 expression. These findings suggest that WWP1 may promote cell proliferation and survival partially through suppressing RNF11-mediated ErbB2 and EGFR downregulation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Abbreviations
- EGFR:
-
epithelial growth factor receptor
- ErbB2:
-
erythroblastic leukemia viral oncogene homolog 2
- GST:
-
glutathione S-transferase
- HECT:
-
homologous to the E6-associated protein C terminus
- IHC:
-
immunohistochemistry
- IP:
-
immunoprecipitation
- mUb:
-
monoubiquitination
- RING:
-
really interesting new gene
- RNF11:
-
RING finger protein 11
- TGF-β:
-
transforming growth factor-β
- WT:
-
wild type
- WWP1:
-
WW domain containing E3 ubiquitin protein ligase 1
References
Anderson LR, Betarbet R, Gearing M, Gulcher J, Hicks AA, Stefansson K et al. (2007). PARK10 candidate RNF11 is expressed by vulnerable neurons and localizes to Lewy bodies in Parkinson disease brain. J Neuropathol Exp Neurol 66: 955–964.
Angers A, Ramjaun AR, McPherson PS . (2004). The HECT domain ligase itch ubiquitinates endophilin and localizes to the trans-Golgi network and endosomal system. J Biol Chem 279: 11471–11479.
Azmi P, Seth A . (2005). RNF11 is a multifunctional modulator of growth factor receptor signalling and transcriptional regulation. Eur J Cancer 41: 2549–2560.
Burger A, Amemiya Y, Kitching R, Seth AK . (2006). Novel RING E3 ubiquitin ligases in breast cancer. Neoplasia 8: 689–695.
Chen C, Matesic LE . (2007). The Nedd4-like family of E3 ubiquitin ligases and cancer. Cancer Metastasis Rev 26: 587–604.
Chen C, Sun X, Guo P, Dong XY, Sethi P, Cheng X et al. (2005). Human Kruppel-like factor 5 is a target of the E3 ubiquitin ligase WWP1 for proteolysis in epithelial cells. J Biol Chem 280: 41553–41561.
Chen C, Sun X, Guo P, Dong XY, Sethi P, Zhou W et al. (2007a). Ubiquitin E3 ligase WWP1 as an oncogenic factor in human prostate cancer. Oncogene 26: 2386–2394.
Chen C, Zhou Z, Ross JS, Zhou W, Dong JT . (2007b). The amplified WWP1 gene is a potential molecular target in breast cancer. Int J Cancer 121: 2834–2841.
Courbard JR, Fiore F, Adelaide J, Borg JP, Birnbaum D, Ollendorff V . (2002). Interaction between two ubiquitin-protein isopeptide ligases of different classes, CBLC and AIP4/ITCH. J Biol Chem 277: 45267–45275.
Flasza M, Nguyen Huu NS, Mazaleyrat S, Clemence S, Villemant C, Clarke R et al. (2006). Regulation of the nuclear localization of the human Nedd4-related WWP1 protein by Notch. Mol Membr Biol 23: 269–276.
Hoeller D, Crosetto N, Blagoev B, Raiborg C, Tikkanen R, Wagner S et al. (2006). Regulation of ubiquitin-binding proteins by monoubiquitination. Nat Cell Biol 8: 163–169.
Jones DC, Wein MN, Oukka M, Hofstaetter JG, Glimcher MJ, Glimcher LH . (2006). Regulation of adult bone mass by the zinc-finger adapter protein Schnurri-3. Science 312: 1223–1227.
Katz M, Shtiegman K, Tal-Or P, Yakir L, Mosesson Y, Harari D et al. (2002). Ligand-independent degradation of epidermal growth factor receptor involves receptor ubiquitylation and Hgs, an adaptor whose ubiquitin-interacting motif targets ubiquitylation by Nedd4. Traffic 3: 740–751.
Kitching R, Wong MJ, Koehler D, Burger AM, Landberg G, Gish G et al. (2003). The RING-H2 protein RNF11 is differentially expressed in breast tumours and interacts with HECT-type E3 ligases. Biochim Biophys Acta 1639: 104–112.
Komuro A, Imamura T, Saitoh M, Yoshida Y, Yamori T, Miyazono K et al. (2004). Negative regulation of transforming growth factor-beta (TGF-beta) signaling by WW domain-containing protein 1 (WWP1). Oncogene 23: 6914–6923.
Laine A, Ronai Z . (2007). Regulation of p53 localization and transcription by the HECT domain E3 ligase WWP1. Oncogene 26: 1477–1483.
Li H, Seth A . (2004). An RNF11: Smurf2 complex mediates ubiquitination of the AMSH protein. Oncogene 23: 1801–1808.
Magnifico A, Ettenberg S, Yang C, Mariano J, Tiwari S, Fang S et al. (2003). WW domain HECT E3s target Cbl RING finger E3s for proteasomal degradation. J Biol Chem 278: 43169–43177.
Marchese A, Raiborg C, Santini F, Keen JH, Stenmark H, Benovic JL . (2003). The E3 ubiquitin ligase AIP4 mediates ubiquitination and sorting of the G protein-coupled receptor CXCR4. Dev Cell 5: 709–722.
Martin-Serrano J, Eastman SW, Chung W, Bieniasz PD . (2005). HECT ubiquitin ligases link viral and cellular PPXY motifs to the vacuolar protein-sorting pathway. J Cell Biol 168: 89–101.
Moren A, Imamura T, Miyazono K, Heldin CH, Moustakas A . (2005). Degradation of the tumor suppressor Smad4 by WW and HECT domain ubiquitin ligases. J Biol Chem 280: 22115–22123.
Mosser EA, Kasanov JD, Forsberg EC, Kay BK, Ney PA, Bresnick EH . (1998). Physical and functional interactions between the transactivation domain of the hematopoietic transcription factor NF-E2 and WW domains. Biochemistry 37: 13686–13695.
Murdaca J, Treins C, Monthouel-Kartmann MN, Pontier-Bres R, Kumar S, Van Obberghen E et al. (2004). Grb10 prevents Nedd4-mediated vascular endothelial growth factor receptor-2 degradation. J Biol Chem 279: 26754–26761.
Plant PJ, Yeger H, Staub O, Howard P, Rotin D . (1997). The C2 domain of the ubiquitin protein ligase Nedd4 mediates Ca2+-dependent plasma membrane localization. J Biol Chem 272: 32329–32336.
Qiu L, Joazeiro C, Fang N, Wang HY, Elly C, Altman Y et al. (2000). Recognition and ubiquitination of Notch by Itch, a hect-type E3 ubiquitin ligase. J Biol Chem 275: 35734–35737.
Rual JF, Venkatesan K, Hao T, Hirozane-Kishikawa T, Dricot A, Li N et al. (2005). Towards a proteome-scale map of the human protein–protein interaction network. Nature 437: 1173–1178.
Seo SR, Lallemand F, Ferrand N, Pessah M, L’Hoste S, Camonis J et al. (2004). The novel E3 ubiquitin ligase Tiul1 associates with TGIF to target Smad2 for degradation. EMBO J 23: 3780–3792.
Shaner NC, Campbell RE, Steinbach PA, Giepmans BN, Palmer AE, Tsien RY . (2004). Improved monomeric red, orange and yellow fluorescent proteins derived from Discosoma sp. red fluorescent protein. Nat Biotechnol 22: 1567–1572.
Shen R, Chen M, Wang YJ, Kaneki H, Xing L, O’Keefe RJ et al. (2006). Smad6 interacts with Runx2 and mediates Smad ubiquitin regulatory factor 1-induced Runx2 degradation. J Biol Chem 281: 3569–3576.
Subramaniam V, Li H, Wong M, Kitching R, Attisano L, Wrana J et al. (2003). The RING-H2 protein RNF11 is overexpressed in breast cancer and is a target of Smurf2 E3 ligase. Br J Cancer 89: 1538–1544.
Vecchione A, Marchese A, Henry P, Rotin D, Morrione A . (2003). The Grb10/Nedd4 complex regulates ligand-induced ubiquitination and stability of the insulin-like growth factor I receptor. Mol Cell Biol 23: 3363–3372.
Verdecia MA, Joazeiro CA, Wells NJ, Ferrer JL, Bowman ME, Hunter T et al. (2003). Conformational flexibility underlies ubiquitin ligation mediated by the WWP1 HECT domain E3 ligase. Mol Cell 11: 249–259.
Wang SE, Narasanna A, Perez-Torres M, Xiang B, Wu FY, Yang S et al. (2006). HER2 kinase domain mutation results in constitutive phosphorylation and activation of HER2 and EGFR and resistance to EGFR tyrosine kinase inhibitors. Cancer Cell 10: 25–38.
Woelk T, Oldrini B, Maspero E, Confalonieri S, Cavallaro E, Di Fiore PP et al. (2006). Molecular mechanisms of coupled monoubiquitination. Nat Cell Biol 8: 1246–1254.
Yarden Y . (2001). The EGFR family and its ligands in human cancer. Signalling mechanisms and therapeutic opportunities. Eur J Cancer 37 (Suppl 4): S3–S8.
Zhang X, Srinivasan SV, Lingrel JB . (2004). WWP1-dependent ubiquitination and degradation of the lung Kruppel-like factor, KLF2. Biochem Biophys Res Commun 316: 139–148.
Acknowledgements
We thank Dr Roger Y Tsien for the mCherry vector. This work was supported in part by a grant from American Cancer Society (Chen C), a grant from the Department of Defense Prostate Cancer Research Program (W81XWH-07-1-0191, Chen C), a grant (BCTR0503705) from the Susan G Komen Breast Cancer Foundation (Chen C), and from the Canadian Breast Cancer Research Alliance special program grant on metastasis to Arun Seth.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on the Oncogene website (http://www.nature.com/onc)
Supplementary information
Rights and permissions
About this article
Cite this article
Chen, C., Zhou, Z., Liu, R. et al. The WW domain containing E3 ubiquitin protein ligase 1 upregulates ErbB2 and EGFR through RING finger protein 11. Oncogene 27, 6845–6855 (2008). https://doi.org/10.1038/onc.2008.288
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2008.288
Keywords
This article is cited by
-
The role of NEDD4 related HECT-type E3 ubiquitin ligases in defective autophagy in cancer cells: molecular mechanisms and therapeutic perspectives
Molecular Medicine (2023)
-
WWP1 E3 ligase at the crossroads of health and disease
Cell Death & Disease (2023)
-
CircWAC induces chemotherapeutic resistance in triple-negative breast cancer by targeting miR-142, upregulating WWP1 and activating the PI3K/AKT pathway
Molecular Cancer (2021)
-
The emerging role of WWP1 in cancer development and progression
Cell Death Discovery (2021)
-
TRIB3-EGFR interaction promotes lung cancer progression and defines a therapeutic target
Nature Communications (2020)