Key Points
-
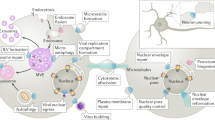
Endocytic trafficking and subsequent recycling of plasma-membrane components is essential for cell viability. They direct the movement of vesicular carriers from the cell surface to the cell interior and back.
-
Endocytic uptake by phagocytosis or macropinocytosis requires different myosin motors that participate in the extension and closure of phagocytic cup pseudopods and their initial transport.
-
Clathrin-mediated endocytosis is tightly linked to the activity of the plus-end directed motor myosin VI, whereas an involvement of a motor(s) in caveolar endocytosis is unclear at present.
-
Microtubule-dependent, minus-end-directed transport definitely requires dynein and its activator, dynactin. The participation of a minus-end-directed kinesin has been suspected, but not proven conclusively.
-
Plus-end-directed motors from several kinesin families also participate in endosomal trafficking along microtubules, leading to prominent bidirectional movement of early and late endosomes as well as lysosomes.
-
Recycling can occur through several pathways, possibly starting at the early endosome step, and requires plus-end-directed microtubule transport. Depending on the cargo in question, transport is powered by members of the kinesin-1, -2 and -3 families.
-
A prominent motor class that is involved in the final steps of delivery to the cell surface is myosin V, although class-I myosins also operate primarily in the cell cortex near the plasma membrane.
-
The cooperation between microtubule and actin motors entails control by small G-proteins and other regulators. Different motors and their regulators can thereby form a complex on the surfaces of trafficking organelles, although this is hypothetical at present.
Abstract
Early in evolution, the diversification of membrane-bound compartments that characterize eukaryotic cells was accompanied by the elaboration of molecular machineries that mediate intercompartmental communication and deliver materials to specific destinations. Molecular motors that move on tracks of actin filaments or microtubules mediate the movement of organelles and transport between compartments. The subjects of this review are the motors that power the transport steps along the endocytic and recycling pathways, their modes of attachment to cargo and their regulation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Schliwa, M. (ed.) Molecular Motors 1–582 (Wiley VCH, Weinheim, 2003).
Soldati, T. Unconventional myosins, actin dynamics and endocytosis: a menage a trois? Traffic 4, 358–366 (2003).
Kaksonen, M., Toret, C. P. & Drubin, D. G. Harnessing actin dynamics for clathrin-mediated endocytosis. Nature Rev. Mol. Cell Biol. 7, 404–414 (2006).
Vallee, R. B., Williams, J. C., Varma, D. & Barnhart, L. E. Dynein: an ancient motor protein involved in multiple modes of transport. J. Neurobiol. 58, 189–200 (2004).
Hirokawa, N. & Takemura, R. Molecular motors and mechanisms of directional transport in neurons. Nature Rev. Neurosci. 6, 201–214 (2005).
Sakato, M. & King, S. M. Design and regulation of the AAA+ microtubule motor dynein. J. Struct. Biol. 146, 58–71 (2004).
Conner, S. D. & Schmid, S. L. Regulated portals of entry into the cell. Nature 422, 37–44 (2003).
Nichols, B. Caveosomes and endocytosis of lipid rafts. J. Cell Sci. 116, 4707–4714 (2003).
Swanson, J. A. et al. A contractile activity that closes phagosomes in macrophages. J. Cell Sci. 112, 307–316 (1999).
Cox, D. et al. Myosin X is a downstream effector of PI(3)K during phagocytosis. Nature Cell Biol. 4, 469–477 (2002).
Olazabal, I. M. et al. Rho-kinase and myosin-II control phagocytic cup formation during CR, but not FcγR, phagocytosis. Curr. Biol. 12, 1413–1418 (2002).
Durrwang, U. et al. Dictyostelium myosin-IE is a fast molecular motor involved in phagocytosis. J. Cell Sci. 119, 550–558 (2006).
Titus, M. A. The role of unconventional myosins in Dictyostelium endocytosis. J. Eukaryot. Microbiol. 47, 191–196 (2000).
Hasson, T. Molecular motors: sensing a function for myosin-VIIa. Curr. Biol. 9, R838–R841 (1999).
Tuxworth, R. I., Stephens, S., Ryan, Z. C. & Titus, M. A. Identification of a myosin VII–talin complex. J. Biol. Chem. 280, 26557–26564 (2005).
Titus, M. A. A conserved role for myosin VII in adhesion. Novartis Found. Symp. 269, 16–34; 223–230 (2005).
Yang, Y., Kovacs, M., Xu, Q., Anderson, J. B. & Sellers, J. R. Myosin VIIB from Drosophila is a high duty ratio motor. J. Biol. Chem. 280, 32061–32068 (2005).
Henn, A. & De La Cruz, E. M. Vertebrate myosin VIIb is a high duty ratio motor adapted for generating and maintaining tension. J. Biol. Chem. 280, 39665–39676 (2005).
Etournay, R. et al. PHR1, an integral membrane protein of the inner ear sensory cells, directly interacts with myosin 1c and myosin VIIa. J. Cell Sci. 118, 2891–2899 (2005).
Araki, N. Role of microtubules and myosins in Fc γ receptor-mediated phagocytosis. Front. Biosci. 11, 1479–1490 (2006).
Ostap, E. M. et al. Dynamic localization of myosin-I to endocytic structures in Acanthamoeba. Cell Motil. Cytoskeleton 54, 29–40 (2003).
Schwarz, E. C., Neuhaus, E. M., Kistler, C., Henkel, A. W. & Soldati, T. Dictyostelium myosin IK is involved in the maintenance of cortical tension and affects motility and phagocytosis. J. Cell Sci. 113, 621–633 (2000).
Tang, N., Lin, T. & Ostap, E. M. Dynamics of myo1c (myosin-Iβ) lipid binding and dissociation. J. Biol. Chem. 277, 42763–42768 (2002).
Clarke, M., Kohler, J., Heuser, J. & Gerisch, G. Endosome fusion and microtubule-based dynamics in the early endocytic pathway of Dictyostelium. Traffic 3, 791–800 (2002). An enlightening series of live observations — by total internal reflection fluorescence microscopy as well as by other microscopy techniques — on macropinocytosis, endosome maturation, fusion and transport on microtubules.
Yang, Z., Vadlamudi, R. K. & Kumar, R. Dynein light chain 1 phosphorylation controls macropinocytosis. J. Biol. Chem. 280, 654–659 (2005).
Kaksonen, M., Toret, C. P. & Drubin, D. G. A modular design for the clathrin- and actin-mediated endocytosis machinery. Cell 123, 305–320 (2005).
Jonsdottir, G. A. & Li, R. Dynamics of yeast myosin I: evidence for a possible role in scission of endocytic vesicles. Curr. Biol. 14, 1604–1609 (2004).
Takeda, T. & Chang, F. Role of fission yeast myosin I in organization of sterol-rich membrane domains. Curr. Biol. 15, 1331–1336 (2005).
Wesche, S., Arnold, M. & Jansen, R. P. The UCS domain protein She4p binds to myosin motor domains and is essential for class I and class V myosin function. Curr. Biol. 13, 715–724 (2003).
Biemesderfer, D., Mentone, S. A., Mooseker, M. & Hasson, T. Expression of myosin VI within the early endocytic pathway in adult and developing proximal tubules. Am. J. Physiol. Renal Physiol. 282, F785–F794 (2002).
Roberts, R. et al. Myosin VI: cellular functions and motor properties. Philos. Trans. R. Soc. Lond. B Biol. Sci. 359, 1931–1944 (2004).
Aschenbrenner, L., Naccache, S. N. & Hasson, T. Uncoated endocytic vesicles require the unconventional myosin, Myo6, for rapid transport through actin barriers. Mol. Biol. Cell 15, 2253–2263 (2004).
Lister, I. et al. A monomeric myosin VI with a large working stroke. EMBO J. 23, 1729–1738 (2004).
Park, H. et al. Full-length myosin VI dimerizes and moves processively along actin filaments upon monomer clustering. Mol. Cell 21, 331–336 (2006). A crisp and splendid demonstration of the cargo-induced dimerization of myosin VI and of its processive movement along actin filaments in large steps.
Tomishige, M., Klopfenstein, D. R. & Vale, R. D. Conversion of Unc104/KIF1A kinesin into a processive motor after dimerization. Science 297, 2263–2267 (2002).
Iwaki, M. et al. Cargo-binding makes a wild-type single-headed myosin-VI move processively. Biophys. J. 90, 3643–3652 (2006).
Reed, B. C. et al. GLUT1CBP(TIP2/GIPC1) interactions with GLUT1 and myosin VI: evidence supporting an adapter function for GLUT1CBP. Mol. Biol. Cell 16, 4183–4201 (2005).
Dance, A. L. et al. Regulation of myosin-VI targeting to endocytic compartments. Traffic 5, 798–813 (2004).
Swiatecka-Urban, A. et al. Myosin VI regulates endocytosis of the cystic fibrosis transmembrane conductance regulator. J. Biol. Chem. 279, 38025–38031 (2004).
Morris, S. M. et al. Myosin VI binds to and localises with Dab2, potentially linking receptor-mediated endocytosis and the actin cytoskeleton. Traffic 3, 331–341 (2002). A study that revealed strong functional evidence for the adaptor-mediated link of myosin VI to clathrin-mediated endocytosis.
Osterweil, E., Wells, D. G. & Mooseker, M. S. A role for myosin VI in postsynaptic structure and glutamate receptor endocytosis. J. Cell Biol. 168, 329–338 (2005).
Allan, V. J., Thompson, H. M. & McNiven, M. A. Motoring around the Golgi. Nature Cell Biol. 4, E236–E242 (2002).
Sahlender, D. A. et al. Optineurin links myosin VI to the Golgi complex and is involved in Golgi organization and exocytosis. J. Cell Biol. 169, 285–295 (2005).
Warner, C. L. et al. Loss of myosin VI reduces secretion and the size of the Golgi in fibroblasts from Snell's waltzer mice. EMBO J. 22, 569–579 (2003).
Pelkmans, L. Viruses as probes for systems analysis of cellular signalling, cytoskeleton reorganization and endocytosis. Curr. Opin. Microbiol. 8, 331–337 (2005).
Pelkmans, L., Puntener, D. & Helenius, A. Local actin polymerization and dynamin recruitment in SV40-induced internalization of caveolae. Science 296, 535–539 (2002).
Tagawa, A. et al. Assembly and trafficking of caveolar domains in the cell: caveolae as stable, cargo-triggered, vesicular transporters. J. Cell Biol. 170, 769–779 (2005).
Rink, J., Ghigo, E., Kalaidzidis, Y. & Zerial, M. Rab conversion as a mechanism of progression from early to late endosomes. Cell 122, 735–749 (2005).
Southwick, F. S., Li, W., Zhang, F., Zeile, W. L. & Purich, D. L. Actin-based endosome and phagosome rocketing in macrophages: activation by the secretagogue antagonists lanthanum and zinc. Cell Motil. Cytoskeleton 54, 41–55 (2003).
Merrifield, C. J. et al. Annexin 2 has an essential role in actin-based macropinocytic rocketing. Curr. Biol. 11, 1136–1141 (2001).
Orth, J. D., Krueger, E. W., Cao, H. & McNiven, M. A. The large GTPase dynamin regulates actin comet formation and movement in living cells. Proc. Natl Acad. Sci. USA 99, 167–172 (2002).
Cordonnier, M. N., Dauzonne, D., Louvard, D. & Coudrier, E. Actin filaments and myosin I α cooperate with microtubules for the movement of lysosomes. Mol. Biol. Cell 12, 4013–4029 (2001).
Salas-Cortes, L. et al. Myosin Ib modulates the morphology and the protein transport within multi-vesicular sorting endosomes. J. Cell Sci. 15, 4823–4832 (2005). Beautifully illustrates the complex interaction and role of class I myosins in endosomal morphology and function.
Kuznetsov, S. A., Langford, G. M. & Weiss, D. G. Actin-dependent organelle movement in squid axoplasm. Nature 356, 722–725 (1992). A seminal paper that described, for the first time, that the same organelle can move on both microtubules and actin filaments.
Lansbergen, G. & Akhmanova, A. Microtubule plus end: a hub of cellular activities. Traffic 7, 499–507 (2006).
Vaughan, K. T. Microtubule plus ends, motors, and traffic of Golgi membranes. Biochim. Biophys. Acta 1744, 316–324 (2005).
Rickard, J. E. & Kreis, T. E. CLIPs for organelle-microtubule interactions. Trends Cell Biol. 6, 178–183 (1996).
Lansbergen, G. et al. Conformational changes in CLIP-170 regulate its binding to microtubules and dynactin localization. J. Cell Biol. 166, 1003–1014 (2004).
Lantz, V. A. & Miller, K. G. A class VI unconventional myosin is associated with a homologue of a microtubule-binding protein, cytoplasmic linker protein-170, in neurons and at the posterior pole of Drosophila embryos. J. Cell Biol. 140, 897–910 (1998).
Xiang, X. A +TIP for a smooth trip. J. Cell Biol. 172, 651–654 (2006).
Bananis, E., Murray, J. W., Stockert, R. J., Satir, P. & Wolkoff, A. W. Regulation of early endocytic vesicle motility and fission in a reconstituted system. J. Cell Sci. 116, 2749–2761 (2003).
Yang, Z., Roberts, E. A. & Goldstein, L. S. Functional analysis of mouse C-terminal kinesin motor KifC2. Mol. Cell. Biol. 21, 2463–2466 (2001).
Bananis, E. et al. Microtubule-dependent movement of late endocytic vesicles in vitro: requirements for dynein and kinesin. Mol. Biol. Cell 15, 3688–3697 (2004).
Valetti, C. et al. Role of dynactin in endocytic traffic: effects of dynamitin overexpression and colocalization with CLIP-170. Mol. Biol. Cell 10, 4107–4120 (1999).
King, S. J., Brown, C. L., Maier, K. C., Quintyne, N. J. & Schroer, T. A. Analysis of the dynein–dynactin interaction in vitro and in vivo. Mol. Biol. Cell 14, 5089–5097 (2003).
Schroer, T. A. Dynactin. Annu. Rev. Cell Dev. Biol. 20, 759–779 (2004).
Levy, J. R. & Holzbaur, E. L. Cytoplasmic dynein/dynactin function and dysfunction in motor neurons. Int. J. Dev. Neurosci. 24, 103–111 (2006).
Harrison, R. E., Bucci, C., Vieira, O. V., Schroer, T. A. & Grinstein, S. Phagosomes fuse with late endosomes and/or lysosomes by extension of membrane protrusions along microtubules: role of Rab7 and RILP. Mol. Cell. Biol. 23, 6494–6506 (2003).
Jordens, I. et al. The Rab7 effector protein RILP controls lysosomal transport by inducing the recruitment of dynein–dynactin motors. Curr. Biol. 11, 1680–1685 (2001).
Cantalupo, G., Alifano, P., Roberti, V., Bruni, C. B. & Bucci, C. Rab-interacting lysosomal protein (RILP): the Rab7 effector required for transport to lysosomes. EMBO J. 20, 683–693 (2001).
Johansson, M., Lehto, M., Tanhuanpaa, K., Cover, T. L. & Olkkonen, V. M. The oxysterol-binding protein homologue ORP1L interacts with Rab7 and alters functional properties of late endocytic compartments. Mol. Biol. Cell 16, 5480–5492 (2005).
Marsman, M. et al. A splice variant of RILP induces lysosomal clustering independent of dynein recruitment. Biochem. Biophys. Res. Commun. 344, 747–756 (2006).
Varma, D., Dujardin, D. L., Stehman, S. A. & Vallee, R. B. Role of the kinetochore/cell cycle checkpoint protein ZW10 in interphase cytoplasmic dynein function. J. Cell Biol. 172, 655–662 (2006).
Hoepfner, S. et al. Modulation of receptor recycling and degradation by the endosomal kinesin KIF16B. Cell 121, 437–450 (2005). Clear evidence for the specific role of a kinesin-3-family motor in early transport steps.
Wedlich-Soldner, R., Straube, A., Friedrich, M. W. & Steinberg, G. A balance of KIF1A-like kinesin and dynein organizes early endosomes in the fungus Ustilago maydis. EMBO J. 21, 2946–2957 (2002).
Lenz, J. H., Schuchardt, I., Straube, A. & Steinberg, G. A dynein loading zone for retrograde endosome motility at microtubule plus-ends. EMBO J. 25, 2275–2286 (2006). Nice demonstration, in a fungal cell model, for a switch between kinesin-powered and dynein-driven movement of endosomes.
Mesngon, M. T. et al. Regulation of cytoplasmic dynein ATPase by Lis1. J. Neurosci. 26, 2132–2139 (2006).
Vallee, R. B. & Tsai, J. W. The cellular roles of the lissencephaly gene LIS1, and what they tell us about brain development. Genes Dev. 20, 1384–1393 (2006).
Brown, C. L. et al. Kinesin-2 is a motor for late endosomes and lysosomes. Traffic 6, 1114–1124 (2005).
Welte, M. A. Bidirectional transport along microtubules. Curr. Biol. 14, R525–R537 (2004).
Ling, S. C., Fahrner, P. S., Greenough, W. T. & Gelfand, V. I. Transport of Drosophila fragile X mental retardation protein-containing ribonucleoprotein granules by kinesin-1 and cytoplasmic dynein. Proc. Natl Acad. Sci. USA 101, 17428–17433 (2004).
He, Y. et al. Role of cytoplasmic dynein in the axonal transport of microtubules and neurofilaments. J. Cell Biol. 168, 697–703 (2005).
Huang, J. D. et al. Direct interaction of microtubule- and actin-based transport motors. Nature 397, 267–270 (1999).
Levi, V., Serpinskaya, A. S., Gratton, E. & Gelfand, V. Organelle transport along microtubules in Xenopus melanophores: evidence for cooperation between multiple motors. Biophys. J. 90, 318–327 (2006).
Ross, J. L., Wallace, K., Shuman, H., Goldman, Y. E. & Holzbaur, E. L. Processive bidirectional motion of dynein–dynactin complexes in vitro. Nature Cell Biol. 8, 562–570 (2006). Reports the stunning observation that in vitro , the dynein–dynactin complex can move bidirectionally on microtubules, although the 'reverse' movement towards the microtubule plus end is less robust.
Liang, Y. et al. Nudel functions in membrane traffic mainly through association with Lis1 and cytoplasmic dynein. J. Cell Biol. 164, 557–566 (2004).
Lebrand, C. et al. Late endosome motility depends on lipids via the small GTPase Rab7. EMBO J. 21, 1289–1300 (2002).
Maxfield, F. R. & McGraw, T. E. Endocytic recycling. Nature Rev. Mol. Cell Biol. 5, 121–132 (2004).
Lakadamyali, M., Rust, M. J. & Zhuang, X. Ligands for clathrin-mediated endocytosis are differentially sorted into distinct populations of early endosomes. Cell 124, 997–1009 (2006).
Lin, S. X., Gundersen, G. G. & Maxfield, F. R. Export from pericentriolar endocytic recycling compartment to cell surface depends on stable, detyrosinated (glu) microtubules and kinesin. Mol. Biol. Cell 13, 96–109 (2002).
Matsushita, M., Tanaka, S., Nakamura, N., Inoue, H. & Kanazawa, H. A novel kinesin-like protein, KIF1Bβ3 is involved in the movement of lysosomes to the cell periphery in non-neuronal cells. Traffic 5, 140–151 (2004).
Wozniak, M. J., Milner, R. & Allan, V. N-terminal kinesins: many and various. Traffic 5, 400–410 (2004).
Nakata, T. & Hirokawa, N. Point mutation of adenosine triphosphate-binding motif generated rigor kinesin that selectively blocks anterograde lysosome membrane transport. J. Cell Biol. 131, 1039–1053 (1995).
Tanaka, Y. et al. Targeted disruption of mouse conventional kinesin heavy chain, kif5B, results in abnormal perinuclear clustering of mitochondria. Cell 93, 1147–1158 (1998).
Santama, N. et al. KIF2β, a new kinesin superfamily protein in non-neuronal cells, is associated with lysosomes and may be implicated in their centrifugal translocation. EMBO J. 17, 5855–5867 (1998).
Moore, A. T. et al. MCAK associates with the tips of polymerizing microtubules. J. Cell Biol. 169, 391–397 (2005).
Semiz, S. et al. Conventional kinesin KIF5B mediates insulin-stimulated GLUT4 movements on microtubules. EMBO J. 22, 2387–2399 (2003).
Imamura, T. et al. Insulin-induced GLUT4 translocation involves protein kinase C-λ-mediated functional coupling between Rab4 and the motor protein kinesin. Mol. Cell. Biol. 23, 4892–4900 (2003).
Huang, J., Imamura, T. & Olefsky, J. M. Insulin can regulate GLUT4 internalization by signaling to Rab5 and the motor protein dynein. Proc. Natl Acad. Sci. USA 98, 13084–13089 (2001).
Bose, A. et al. Unconventional myosin Myo1c promotes membrane fusion in a regulated exocytic pathway. Mol. Cell. Biol. 24, 5447–5458 (2004).
Rudolf, R. et al. Myosin Va facilitates the distribution of secretory granules in the F-actin rich cortex of PC12 cells. J. Cell Sci. 116, 1339–1348 (2003).
Lang, T. et al. Role of actin cortex in the subplasmalemmal transport of secretory granules in PC-12 cells. Biophys. J. 78, 2863–2877 (2000). Reconciled the contradictory views about the role of the actin cytoskeleton in exocytosis and replenishment of the releasable pool of granules.
Brown, J. R., Stafford, P. & Langford, G. M. Short-range axonal/dendritic transport by myosin-V: a model for vesicle delivery to the synapse. J. Neurobiol. 58, 175–188 (2004).
Shupliakov, O. et al. Impaired recycling of synaptic vesicles after acute perturbation of the presynaptic actin cytoskeleton. Proc. Natl Acad. Sci. USA 99, 14476–14481 (2002).
Bridgman, P. C. Myosin Va movements in normal and dilute-lethal axons provide support for a dual filament motor complex. J. Cell Biol. 146, 1045–1060 (1999).
Watanabe, M. et al. Myosin-Va regulates exocytosis through the submicromolar Ca2+-dependent binding of syntaxin-1A. Mol. Biol. Cell 16, 4519–4530 (2005).
Wakabayashi, Y., Dutt, P., Lippincott-Schwartz, J. & Arias, I. M. Rab11a and myosin Vb are required for bile canalicular formation in WIF-B9 cells. Proc. Natl Acad. Sci. USA 102, 15087–15092 (2005).
Lindsay, A. J. & McCaffrey, M. W. Rab11-FIP2 functions in transferrin recycling and associates with endosomal membranes via its COOH-terminal domain. J. Biol. Chem. 277, 27193–27199 (2002).
Fan, G. H., Lapierre, L. A., Goldenring, J. R., Sai, J. & Richmond, A. Rab11-family interacting protein 2 and myosin Vb are required for CXCR2 recycling and receptor-mediated chemotaxis. Mol. Biol. Cell 15, 2456–2469 (2004).
Volpicelli, L. A., Lah, J. J., Fang, G., Goldenring, J. R. & Levey, A. I. Rab11a and myosin Vb regulate recycling of the M4 muscarinic acetylcholine receptor. J. Neurosci. 22, 9776–9784 (2002).
Leiva, N., Pavarotti, M., Colombo, M. I. & Damiani, M. T. Reconstitution of recycling from the phagosomal compartment in streptolysin O-permeabilized macrophages: role of Rab11. Exp. Cell Res. 312, 1843–1855 (2006).
Hales, C. M., Vaerman, J. P. & Goldenring, J. R. Rab11 family interacting protein 2 associates with Myosin Vb and regulates plasma membrane recycling. J. Biol. Chem. 277, 50415–50421 (2002).
Hobdy-Henderson, K. C., Hales, C. M., Lapierre, L. A., Cheney, R. E. & Goldenring, J. R. Dynamics of the apical plasma membrane recycling system during cell division. Traffic 4, 681–693 (2003).
Stachelek, S. J. et al. Real-time visualization of processive myosin 5a-mediated vesicle movement in living astrocytes. J. Biol. Chem. 276, 35652–35659 (2001).
Rodriguez, O. C. & Cheney, R. E. Human myosin-Vc is a novel class V myosin expressed in epithelial cells. J. Cell Sci. 115, 991–1004 (2002).
Yan, Q. et al. CART: an Hrs–actinin-4–BERP–myosin V protein complex required for efficient receptor recycling. Mol. Biol. Cell 16, 2470–2482 (2005).
Bose, A. et al. Glucose transporter recycling in response to insulin is facilitated by myosin Myo1c. Nature 420, 821–824 (2002).
Huber, L. A. et al. Both calmodulin and the unconventional myosin Myr4 regulate membrane trafficking along the recycling pathway of MDCK cells. Traffic 1, 494–503 (2000).
Durrbach, A., Raposo, G., Tenza, D., Louvard, D. & Coudrier, E. Truncated brush border myosin I affects membrane traffic in polarized epithelial cells. Traffic 1, 411–424 (2000).
Neuhaus, E. M. & Soldati, T. A myosin I is involved in membrane recycling from early endosomes. J. Cell Biol. 150, 1013–1026 (2000).
Barile, M. et al. Large-scale protein identification in intracellular aquaporin-2 vesicles from renal inner medullary collecting duct. Mol. Cell. Proteomics 4, 1095–1106 (2005).
Togo, T. & Steinhardt, R. A. Nonmuscle myosin IIA and IIB have distinct functions in the exocytosis-dependent process of cell membrane repair. Mol. Biol. Cell 15, 688–695 (2004).
Neco, P. et al. New roles of myosin II during vesicle transport and fusion in chromaffin cells. J. Biol. Chem. 279, 27450–27457 (2004).
Jerdeva, G. V. et al. Actin and non-muscle myosin II facilitate apical exocytosis of tear proteins in rabbit lacrimal acinar epithelial cells. J. Cell Sci. 118, 4797–4812 (2005).
Polo-Parada, L., Plattner, F., Bose, C. & Landmesser, L. T. NCAM 180 acting via a conserved C-terminal domain and MLCK is essential for effective transmission with repetitive stimulation. Neuron 46, 917–931 (2005).
Takagishi, Y. et al. Localization of myosin II and V isoforms in cultured rat sympathetic neurones and their potential involvement in presynaptic function. J. Physiol. 569, 195–208 (2005).
Rose, S. D. et al. Myosins II and V in chromaffin cells: myosin V is a chromaffin vesicle molecular motor involved in secretion. J. Neurochem. 85, 287–298 (2003).
Schliwa, M. & Woehlke, G. Molecular motors. Nature 422, 759–765 (2003).
Kull, F. J., Sablin, E. P., Lau, R., Fletterick, R. J. & Vale, R. D. Crystal structure of the kinesin motor domain reveals a structural similarity to myosin. Nature 380, 550–555 (1996).
Samso, M. & Koonce, M. P. 25 Å resolution structure of a cytoplasmic dynein motor reveals a seven-member planar ring. J. Mol. Biol. 340, 1059–1072 (2004).
Takahashi, Y., Edamatsu, M. & Toyoshima, Y. Y. Multiple ATP-hydrolyzing sites that potentially function in cytoplasmic dynein. Proc. Natl Acad. Sci. USA 101, 12865–12869 (2004).
Burgess, S. A., Walker, M. L., Sakakibara, H., Knight, P. J. & Oiwa, K. Dynein structure and power stroke. Nature 421, 715–718 (2003).
Hancock, W. O. & Howard, J. in Molecular Motors (ed. Schliwa, M.) 243–269 (Wiley VCH, Weinheim, 2003).
Geissler, H., Ullmann, R. & Soldati, T. The tail domain of myosin M catalyses nucleotide exchange on Rac1 GTPases and can induce actin-driven surface protrusions. Traffic 1, 399–410 (2000).
Oishi, N., Adachi, H. & Sutoh, K. Novel Dictyostelium unconventional myosin, MyoM, has a putative RhoGEF domain. FEBS Lett. 474, 16–22 (2000).
Foth, B. J., Goedecke, M. C. & Soldati, D. New insights into myosin evolution and classification. Proc. Natl Acad. Sci. USA 103, 3681–3686 (2006).
Kamal, A., Stokin, G. B., Yang, Z., Xia, C. H. & Goldstein, L. S. Axonal transport of amyloid precursor protein is mediated by direct binding to the kinesin light chain subunit of kinesin-I. Neuron 28, 449–459 (2000).
Lazarov, O. et al. Axonal transport, amyloid precursor protein, kinesin-1, and the processing apparatus: revisited. J. Neurosci. 25, 2386–2395 (2005).
Muresan, V. et al. Dynactin-dependent, dynein-driven vesicle transport in the absence of membrane proteins: a role for spectrin and acidic phospholipids. Mol. Cell 7, 173–183 (2001).
Koushika, S. P. et al. Mutations in Caenorhabditis elegans cytoplasmic dynein components reveal specificity of neuronal retrograde cargo. J. Neurosci. 24, 3907–3916 (2004).
Fuchs, E., Short, B. & Barr, F. A. Assay and properties of rab6 interaction with dynein–dynactin complexes. Methods Enzymol. 403, 607–618 (2005).
Nascimento, A. A., Roland, J. T. & Gelfand, V. I. Pigment cells: a model for the study of organelle transport. Annu. Rev. Cell Dev. Biol. 19, 469–491 (2003).
Deacon, S. W. et al. Dynactin is required for bidirectional organelle transport. J. Cell Biol. 160, 297–301 (2003). Shows that dynactin can interact with dynein and kinesin-2 on melanosomes.
Kashina, A. S. et al. Protein kinase A, which regulates intracellular transport, forms complexes with molecular motors on organelles. Curr. Biol. 14, 1877–1881 (2004).
Gross, S. P. et al. Interactions and regulation of molecular motors in Xenopus melanophores. J. Cell Biol. 156, 855–865 (2002).
Wu, X. S. et al. Identification of an organelle receptor for myosin-Va. Nature Cell Biol. 4, 271–278 (2002).
Wu, X. S., Tsan, G. L. & Hammer, J. A. 3rd. Melanophilin and myosin Va track the microtubule plus end on EB1. J. Cell Biol. 171, 201–207 (2005). Clear evidence for the delivery mechanism from the actin to the microtubule transport system.
El-Amraoui, A. et al. MyRIP, a novel Rab effector, enables myosin VIIa recruitment to retinal melanosomes. EMBO Rep. 3, 463–470 (2002).
Desnos, C. et al. Rab27A and its effector MyRIP link secretory granules to F-actin and control their motion towards release sites. J. Cell Biol. 163, 559–570 (2003).
Hannah, M. J. et al. Weibel–Palade bodies recruit Rab27 by a content-driven, maturation-dependent mechanism that is independent of cell type. J. Cell Sci. 116, 3939–3948 (2003).
Chen, X. et al. Organellar proteomics: analysis of pancreatic zymogen granule membranes. Mol. Cell. Proteomics 5, 306–312 (2006).
Lawrence, C. J. et al. A standardized kinesin nomenclature. J. Cell Biol. 167, 19–22 (2004).
Asai, D. J. & Wilkes, D. E. The dynein heavy chain family. J. Eukaryot. Microbiol. 51, 23–29 (2004).
Acknowledgements
Research is supported by grants from the UK Biotechnology and Biological Sciences Research Council, the Wellcome Trust and the Swiss National Science Foundation to T.S., and the Deutsche Forschungsgemeinschaft, the Friedrich–Baur–Stiftung and the Fonds der Chemischen Industrie to M.S. Owing to space limitations, not all of the relevant references could be cited.
Author information
Authors and Affiliations
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Glossary
- Endocytosis
-
A plasma-membrane-associated process in which a eukaryotic cell engulfs extracellular fluid or particles.
- Phagocytosis
-
A form of endocytosis in which a eukaryotic cell engulfs large particles, such as bacteria.
- Pinocytosis
-
A form of endocytosis in which a eukaryotic cell engulfs extracellular fluid and solutes.
- Macropinocytosis
-
A form of pinocytosis, morphologically and mechanistically related to phagocytosis, by which cells form large membrane vesicles.
- Clathrin-mediated endocytosis
-
A form of pinocytosis, often also referred to as receptor-mediated endocytosis, in which the invagination of the endocytic vesicle is driven by the clathrin coat.
- Caveolae-mediated endocytosis
-
A form of pinocytosis that is driven by a coat made of the protein caveolin.
- Clathrin- and caveolae-independent endocytosis
-
A form (or forms) of uptake that is revealed when the two other endocytosis pathways are blocked.
- Lamellipodia
-
Sheet-like plasma-membrane protrusions that are formed by actin polymerization at the leading edge of motile cells.
- Phagocytic cup
-
A bowl-shaped lamellipodia-like protrusion that forms around particles during phagocytic uptake.
- Processivity
-
The capability of a single motor to move for long distances without dissociating from the track.
- Acanthamoeba
-
A genus of small, highly motile soil amoebae that are frequently used for studies of actin-binding proteins and cell locomotion. Some pathogenic forms are known.
- Minus end (plus end) direction
-
Both actin filaments and microtubules demonstrate polarity, and are assembled from monomers that are added at a high rate at one end (the plus end) and at a much slower rate at the other (the minus end). Motors can transport cargo in either direction, processes that are referred to as plus-end-directed and minus-end-directed transport.
- Snell's waltzer phenotype
-
A mouse mutation in myosin VI that arose spontaneously at the Jackson laboratory in the 1960s. It affects inner ear structure and leads to deafness and vestibular dysfunction, causing the mice to circle.
- Caveosomes
-
Endocytic vesicles enriched for the protein caveolin-1 formed from caveolae, flask-shaped pits in the membrane that resemble a cave.
- RAB
-
One family of the large superfamily of small GTP-binding proteins involved in a myriad of cellular functions. RAB proteins are best known for their role in the timing of vesicle fusion.
- Actin comet tail
-
A network of actin filaments that is assembled from one end of an intracellular bacterium (such as Listeria or Shigella) or a cytoplasmic vesicle and that takes on the form of a comet tail. Constant actin polymerization at the surface pushes the bacteria or vesicles through the cytoplasm.
- Melanophore
-
A class of pigment-containing cell that is responsible for the generation of skin and eye colour. The pigment melanin (a tyrosine-polymer) is concentrated in vesicles (melanosomes) that can be translocated in the cell, causing colour change.
- Cell cortex
-
The zone of the cell periphery, directly under the plasma membrane, where the actin cytoskeleton forms a dense meshwork.
- CLIP
-
(Cytoplasmic linker protein). These were originally thought to load endocytic vesicles onto the plus ends of microtubules. CLIPs are part of the microtubule-plus-end-tracking complex (+TIPs) and are associated with the distal ends of microtubules.
- Hyphal apex
-
Hyphae are long, branching filaments that are mostly found in the fungi that form the mycelium, the vegetative network below the ground. Hyphae only grow at the tip, and the hyphal apex contains a growth-related vesicular organelle cluster called Spitzenkörper (tip body).
- Rigor mutant
-
A dominant-negative mutation of the ATP-binding domain in a motor that locks it irreversibly to its cytoskeletal partner.
- Syntaxin-1a
-
A form of SNARE (soluble N-ethylmaleimide-sensitive factor attachment protein receptor) protein with a single transmembrane domain that participates in exocytosis. Its C-terminal domain is part of the core SNARE complex, which mediates membrane fusion.
- Astrocytes
-
Star-shaped glial cells in the brain that interact with neurons in multiple ways. They are identified by the expression of glial fibrillary acidic protein (a type of intermediate filament protein) and they outnumber neurons ten to one.
Rights and permissions
About this article
Cite this article
Soldati, T., Schliwa, M. Powering membrane traffic in endocytosis and recycling. Nat Rev Mol Cell Biol 7, 897–908 (2006). https://doi.org/10.1038/nrm2060
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrm2060
This article is cited by
-
Morphofunctional analysis of fibroblast-like synoviocytes in human rheumatoid arthritis and mouse collagen-induced arthritis
Advances in Rheumatology (2023)
-
Shear Stress Increases V–H\(^{+}\)-ATPase and Acidic Vesicle Number Density, and p-mTORC2 Activation in Prostate Cancer Cells
Cellular and Molecular Bioengineering (2020)
-
Expression of S100 proteins is associated with HBV intrauterine transmission
Archives of Gynecology and Obstetrics (2020)
-
Dopey1-Mon2 complex binds to dual-lipids and recruits kinesin-1 for membrane trafficking
Nature Communications (2019)
-
Slow Release of HIV-1 Protein Nef from Vesicle-like Structures Is Inhibited by Cytosolic Calcium Elevation in Single Human Microglia
Molecular Neurobiology (2019)