Key Points
-
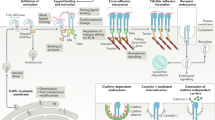
As the main surface receptors that connect cells to the extracellular matrix (ECM), integrins control cell adhesion and migration — the fundamental cell behaviours that underlie development, immune responses and tumorigenesis in animals. As the essential link between ECM and cytoskeleton, integrins relay signals from the ECM to prompt intracellular signal cascades as well as to reshape cell topology in a process termed 'outside–in' signalling. But intracellular interactions can determine the affinity of integrins for their ligands through 'inside–out' signalling.
-
Ras proteins are small GTPases that alternate between GTP-bound and GDP-bound forms, which correspond to their active and inactive conformations, respectively. Ras proteins impart profound effects on the affinity and avidity of integrins. Ras, R-ras and Rap1 are the best studied in this respect.
-
H-ras can either suppress or activate integrin, depending on cellular context and the type of integrin it affects. Raf1 and extracellular signal-regulated kinase (ERK)/mitogen-activated protein kinase (MAPK) might mediate the suppression of integrins by Ras under certain circumstances, whereas phosphatidylinositol 3-kinase (PI3K) could enable Ras to activate integrins.
-
R-ras is an activator of integrins that can convert suspension cells into highly adherent ones. R-ras employs PI3K to effect its activation of integrins in haematopoietic cells, but not in fibroblasts.
-
Like R-ras, Rap1 is an integrin activator. Rap1 can maintain integrins in their active conformation and can promote integrin clustering to enhance avidity. RapL, a newly identified Rap1 effector, might connect Rap1 and integrin in lymphocytes.
-
There is substantial crosstalk among Ras proteins in their regulation of integrin. R-ras counters the suppressive effect of Ras on integrins in fibroblasts. R-ras might also activate integrins through Rap1.
Abstract
Integrins are cell-surface receptors that mediate and coordinate cellular responses to the extracellular matrix (ECM). Cellular signalling pathways can regulate cell adhesion by altering the affinity and avidity of integrins for ECM. The Ras family of small G proteins, which includes H-ras, R-ras and Rap, are important elements in cellular signalling pathways that control integrin function.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
The C. elegans Sequencing Consortium. Genome sequence of the nematode C. elegans: a platform for investigating biology. Science 282, 2012–2018 (1998).
Adams, M. D. et al. The genome sequence of Drosophila melanogaster. Science 287, 2185–2195 (2000).
Cheresh, D. A. & Mecham, R. P. Integrins: molecular and biological responses to the extracellular matrix. (Academic Press, London, 1994).
Haas, T. A. & Plow, E. F. Integrin-ligand interactions: a year in review. Curr. Opin. Cell. Biol. 6, 656–662 (1994).
Loftus, J. C., Smith, J. W. & Ginsberg, M. H. Integrin-mediated cell adhesion: the extracellular face. J. Biol. Chem. 269, 25235–25238 (1994).
Ginsberg, M. H., Loftus, J. C. & Plow, E. F. Common and ligand-specific integrin recognition mechanisms. Chem. Immunol. 50, 75–88 (1991).
Liu, S., Calderwood, D. A. & Ginsberg, M. H. Integrin cytoplasmic domain-binding proteins. J. Cell Sci. 113, 3563–3571 (2000).
Liddington, R. C. & Ginsberg, M. H. Integrin activation takes shape. J. Cell Biol. 158, 833–839 (2002).
Hughes, P. E. et al. Breaking the integrin hinge. A defined structural constraint regulates integrin signaling. J. Biol. Chem. 271, 6571–6574 (1996).
Takagi, J., Erickson, H. P. & Springer, T. A. C-terminal opening mimics 'inside-out' activation of integrin α5β1 . Nature Struct. Biol. 8, 412–416 (2001).
Shimaoka, M., Takagi, J. & Springer, T. A. Conformational regulation of integrin structure and function. Annu. Rev. Biophys. Biomol. Struct. 31, 485–516 (2002).
Reuther, G. W. & Der, C. J. The Ras branch of small GTPases: Ras family members don't fall far from the tree. Curr. Opin. Cell Biol. 12, 157–165 (2000).
Lee, C. H., Della, N. G., Chew, C. E. & Zack, D. J. Rin, a neuron-specific and calmodulin-binding small G-protein, and Rit define a novel subfamily of ras proteins. J. Neurosci. 16, 6784–6794 (1996).
Shao, H., Kadono-Okuda, K., Finlin, B. S. & Andres, D. A. Biochemical characterization of the Ras-related GTPases Rit and Rin. Arch. Biochem. Biophys. 371, 207–219 (1999).
Shields, J. M., Pruitt, K., McFall, A., Shaub, A. & Der, C. J. Understanding Ras: 'it ain't over 'til it's over'. Trends Cell. Biol. 10, 147–154 (2000).
Kitayama, H., Sugimoto, Y., Matsuzaki, T., Ikawa, Y. & Noda, M. A ras-related gene with transformation suppressor activity. Cell 56, 77–84 (1989).
Hughes, P. E. et al. Suppression of integrin activation: a novel function of a Ras/Raf-initiated MAP kinase pathway. Cell 88, 521–530 (1997).
Hughes, P. E. et al. Suppression of integrin activation by activated Ras or Raf does not correlate with bulk activation of ERK MAP kinase. Mol. Biol. Cell 13, 2256–2265 (2002).
Kashiwagi, H. et al. Affinity modulation of platelet integrin αIIbβ3 by β3-endonexin, a selective binding partner of the β3 integrin cytoplasmic tail. J. Cell Biol. 137, 1433–1443 (1997).
Sechler, J. L., Cumiskey, A. M., Gazzola, D. M. & Schwarzbauer, J. E. A novel RGD-independent fibronectin assembly pathway initiated by α4β1 integrin binding to the alternatively spliced V region. J. Cell Sci. 113, 1491–1498 (2000).
Liu, Z. J. et al. A novel role for H-Ras in the regulation of very late antigen-4 integrin and VCAM-1 via c-Myc-dependent and -independent mechanisms. J. Immunol. 163, 4901–4908 (1999).
Shibayama, H. et al. H-Ras is involved in the inside-out signaling pathway of interleukin-3-induced integrin activation. Blood 93, 1540–1548 (1999).
Fujimoto, H. et al. Down-regulation of α6 integrin, an anti-oncogene product, by functional cooperation of H-Ras and c-Myc. Genes Cells 6, 337–343 (2001).
Myou, S. et al. Blockade of focal clustering and active conformation in β2-integrin-mediated adhesion of eosinophils to intercellular adhesion molecule-1 caused by transduction of HIV TAT-dominant negative Ras. J. Immunol. 169, 2670–2676 (2002).
Tanaka, Y. et al. H-Ras signals to cytoskeletal machinery in induction of integrin-mediated adhesion of T cells. J. Immunol. 163, 6209–6216 (1999).
Sethi, T., Ginsberg, M. H., Downward, J. & Hughes, P. E. The small GTP-binding protein R-Ras can influence integrin activation by antagonizing a Ras/Raf-initiated integrin suppression pathway. Mol. Biol. Cell 10, 1799–1809 (1999).
Kinashi, T. et al. Distinct mechanisms of α5β1 integrin activation by Ha-Ras and R-Ras. J. Biol. Chem. 275, 22590–22596 (2000).
Ramos, J. W., Kojima, T. K., Hughes, P. E., Fenczik, C. A. & Ginsberg, M. H. The death effector domain of PEA-15 is involved in its regulation of integrin activation. J. Biol. Chem. 273, 33897–33900 (1998).
Matter, M. L., Ginsberg, M. H. & Ramos, J. W. Identification of cell signaling molecules by expression cloning. Sci. STKE 103, PL9 (2001).
Kitsberg, D. et al. Knock-out of the neural death effector domain protein PEA-15 demonstrates that its expression protects astrocytes from TNFα-induced apoptosis. J. Neurosci. 19, 8244–8251 (1999).
Formstecher, E. et al. PEA-15 mediates cytoplasmic sequestration of ERK MAP kinase. Dev. Cell. 1, 239–250 (2001).
Zhang, Z., Vuori, K., Wang, H., Reed, J. C. & Ruoslahti, E. Integrin activation by R-ras. Cell 85, 61–69 (1996). Demonstrated for the first time the involvement of R-ras in intergin regulation by converting suspension cells into adherent cells with R-ras.
Keely, P. J., Rusyn, E. V., Cox, A. D. & Parise, L. V. R-Ras signals through specific integrin α cytoplasmic domains to promote migration and invasion of breast epithelial cells. J. Cell. Biol. 145, 1077–1088 (1999).
Self, A. J., Caron, E., Paterson, H. F. & Hall, A. Analysis of R-Ras signalling pathways. J. Cell. Sci. 114, 1357–1366 (2001).
Ivins, J. K., Yurchenco, P. D. & Lander, A. D. Regulation of neurite outgrowth by integrin activation. J. Neurosci. 20, 6551–6560 (2000).
Caron, E., Self, A. J. & Hall, A. The GTPase Rap1 controls functional activation of macrophage integrin αMβ2 by LPS and other inflammatory mediators. Curr. Biol. 10, 974–978 (2000).
Wang, B., Zou, J. X., Ek-Rylander, B. & Ruoslahti, E. R-Ras contains a proline-rich site that binds to SH3 domains and is required for integrin activation by R-Ras. J. Biol. Chem. 275, 5222–5227 (2000).
Hansen, M. et al. R-Ras C-terminal sequences are sufficient to confer R-Ras specificity to H-Ras. Oncogene 21, 4448–4461 (2002).
Oertli, B. et al. The effector loop and prenylation site of R-Ras are involved in the regulation of integrin function. Oncogene 19, 4961–4969 (2000).
Berrier, A. L., Mastrangelo, A. M., Downward, J., Ginsberg, M. & LaFlamme, S. E. Activated R-ras, Rac1, PI 3-kinase and PKCε can each restore cell spreading inhibited by isolated integrin β1 cytoplasmic domains. J. Cell Biol. 151, 1549–1560 (2000).
Nimnual, A. S., Yatsula, B. A. & Bar-Sagi, D. Coupling of Ras and Rac guanosine triphosphatases through the Ras exchanger Sos. Science 279, 560–563 (1998).
Han, J. et al. Role of substrates and products of PI 3-kinase in regulating activation of Rac-related guanosine triphosphatases by Vav. Science 279, 558–560 (1998).
Michiels, F. et al. Regulated membrane localization of Tiam1, mediated by the NH2-terminal pleckstrin homology domain, is required for Rac-dependent membrane ruffling and c-Jun NH2-terminal kinase activation. J. Cell Biol. 137, 387–398 (1997).
Storz, P. & Toker, A. 3′-phosphoinositide-dependent kinase-1 (PDK-1) in PI3-kinase signaling. Front. Biosci. 7, d886–d902 (2002).
Cenni, V. et al. Regulation of novel protein kinase Cε by phosphorylation. Biochem. J. 363, 537–545 (2002).
Tsukamoto, N., Hattori, M., Yang, H., Bos, J. L. & Minato, N. Rap1 GTPase-activating protein SPA-1 negatively regulates cell adhesion. J. Biol. Chem. 274, 18463–18469 (1999).
Katagiri, K. et al. Rap1 is a potent activation signal for leukocyte function-associated antigen 1 distinct from protein kinase C and phosphatidylinositol-3-OH kinase. Mol. Cell. Biol. 20, 1956–1969 (2000). Provided the direct evidence for Rap1 involvement in integrin-mediated cell adhesion.
Shimonaka, M. et al. Rap1 translates chemokine signals to integrin activation, cell polarization, and motility across vascular endothelium under flow. J. Cell. Biol. 161, 417–427 (2003).
Katagiri, K., Hattori, M., Minato, N. & Kinashi, T. Rap1 functions as a key regulator of T-cell and antigen-presenting cell interactions and modulates T-cell responses. Mol. Cell. Biol. 22, 1001–1015 (2002).
Enserink, J. M. et al. A novel Epac-specific cAMP analogue demonstrates independent regulation of Rap1 and ERK. Nature Cell. Biol. 4, 901–906 (2002).
Arai, A. et al. Rap1 is activated by erythropoietin or interleukin-3 and is involved in regulation of β1 integrin-mediated hematopoietic cell adhesion. J. Biol. Chem. 276, 10453–10462 (2001).
Bos, J. L., de Rooij, J. & Reedquist, K. A. Rap1 signalling: adhering to new models. Nature Rev. Mol. Cell. Biol. 2, 369–377 (2001).
Fenczik, C. A. et al. Distinct domains of CD98hc regulate integrins and amino acid transport. J. Biol. Chem. 276, 8746–8752 (2001).
Fenczik, C. A., Sethi, T., Ramos, J. W., Hughes, P. E. & Ginsberg, M. H. Complementation of dominant suppression implicates CD98 in integrin activation. Nature 390, 81–85 (1997).
Suga, K. et al. CD98 induces LFA-1-mediated cell adhesion in lymphoid cells via activation of Rap1. FEBS Lett. 489, 249–253 (2001).
Reedquist, K. A. et al. The small GTPase, Rap1, mediates CD31-induced integrin adhesion. J. Cell Biol. 148, 1151–1158 (2000). Provided direct evidence that Rap1 is involved in integrin-mediated adhesion downstream of CD31.
Sebzda, E., Bracke, M., Tugal, T., Hogg, N. & Cantrell, D. A. Rap1A positively regulates T cells via integrin activation rather than inhibiting lymphocyte signaling. Nature Immunol. 3, 251–258 (2002). Provided in vivo evidence that Rap1 stimulates the immune response as opposed to causing anergy and suggested that Rap1 acts through stimulating LFA-1-mediated adhesion.
Amsen, D., Kruisbeek, A., Bos, J. L. & Reedquist, K. Activation of the Ras-related GTPase Rap1 by thymocyte TCR engagement and during selection. Eur. J. Immunol. 30, 2832–2841 (2000).
Stone, J. D. et al. Aberrant TCR-mediated signaling in CD45-null thymocytes involves dysfunctional regulation of Lck, Fyn, TCR-ζ, and ZAP-70. J. Immunol. 158, 5773–5782 (1997).
Tang, Q. et al. The Src family kinase Fyn mediates signals induced by TCR antagonists. J. Immunol. 168, 4480–4487 (2002).
Naramura, M. et al. c-Cbl and Cbl-b regulate T cell responsiveness by promoting ligand-induced TCR down-modulation. Nature Immunol. 3, 1192–1199 (2002).
Ohba, Y. et al. Requirement for C3G-dependent Rap1 activation for cell adhesion and embryogenesis. EMBO J. 20, 3333–3341 (2001).
Rangarajan, S. et al. Cyclic AMP induces integrin-mediated cell adhesion through Epac and Rap1 upon stimulation of the β2-adrenergic receptor. J. Cell Biol. 160, 487–493 (2003). Demonstrated for the first time the requirement of endogenous Rap for integrin activation by the use of 8-pCPT-2′- O -Me-cAMP, a specific activator of the RapGEF Epac.
Bertoni, A. et al. Relationships between Rap1b, affinity modulation of integrin αIIbβ3, and the actin cytoskeleton. J. Biol. Chem. 277, 25715–25721 (2002). Demonstrated the requirement for Rap1 in maintaining integrin in an active conformation.
de Bruyn, K. M., Rangarajan, S., Reedquist, K. A., Figdor, C. G. & Bos, J. L. The small GTPase Rap1 is required for Mn(2+)- and antibody-induced LFA-1- and VLA-4-mediated cell adhesion. J. Biol. Chem. 277, 29468–29476 (2002).
Katagiri, K., Maeda, A., Shimonaka, M. & Kinashi, T. RAPL, a Rap1-binding molecule that mediates Rap1-induced adhesion through spatial regulation of LFA-1. Nature Immunol. 4, 741–748 (2003). Identified Nore1/RapL as the Rap effector that might provide Rap with a direct link to integrin activation.
Tommasi, S. et al. RASSF3 and NORE1: identification and cloning of two human homologues of the putative tumor suppressor gene RASSF1. Oncogene 21, 2713–2720 (2002).
Ohba, Y., Kurokawa, K. & Matsuda, M. Mechanism of the spatio–temporal regulation of Ras and Rap1. EMBO J. 22, 859–869 (2003).
Marte, B. M., Rodriguez-Viciana, P., Wennstrom, S., Warne, P. H. & Downward, J. R-Ras can activate the phosphoinositide 3-kinase but not the MAP kinase arm of the Ras effector pathways. Curr. Biol. 7, 63–70 (1997).
Gao, X. et al. Identification and characterization of RA-GEF-2, a Rap guanine nucleotide exchange factor that serves as a downstream target of M-Ras. J. Biol. Chem. 276, 42219–42225 (2001).
O'Toole, T. E. et al. Integrin cytoplasmic domains mediate inside-out signal transduction. J. Cell Biol. 124, 1047–1059 (1994).
Calderwood, D. A. et al. The phosphotyrosine binding-like domain of talin activates integrins. J. Biol. Chem. 277, 21749–21758 (2002).
Brown, N. H. et al. Talin is essential for integrin function in Drosophila. Dev. Cell 3, 569–579 (2002).
Yan, B., Calderwood, D. A., Yaspan, B. & Ginsberg, M. H. Calpain cleavage promotes talin binding to the β3 integrin cytoplasmic domain. J. Biol. Chem. 276, 28164–28170 (2001).
Martel, V. et al. Conformation, localization, and integrin binding of talin depend on its interaction with phosphoinositides. J. Biol. Chem. 276, 21217–21227 (2001).
Manes, S. et al. Membrane raft microdomains in chemokine receptor function. Semin. Immunol. 13, 147–157 (2001).
Pande, G. The role of membrane lipids in regulation of integrin functions. Curr. Opin. Cell. Biol. 12, 569–574 (2000).
Zhao, J., Kung, H. F. & Manne, V. Farnesylation of p21 Ras proteins in Xenopus oocytes. Cell. Mol. Biol. Res. 40, 313–321 (1994).
Rowell, C. A., Kowalczyk, J. J., Lewis, M. D. & Garcia, A. M. Direct demonstration of geranylgeranylation and farnesylation of Ki-Ras in vivo. J. Biol. Chem. 272, 14093–14097 (1997).
Dong, D. L., Liu, R., Sherlock, R., Wigler, M. H. & Nestler, H. P. Molecular forceps from combinatorial libraries prevent the farnesylation of Ras by binding to its carboxyl terminus. Chem. Biol. 6, 133–141 (1999).
Boyartchuk, V. L., Ashby, M. N. & Rine, J. Modulation of Ras and a-factor function by carboxyl-terminal proteolysis. Science 275, 1796–1800 (1997).
Hancock, J. F., Cadwallader, K. & Marshall, C. J. Methylation and proteolysis are essential for efficient membrane binding of prenylated p21K-ras(B). EMBO J. 10, 641–646 (1991).
Volker, C. & Stock, J. B. Carboxyl methylation of Ras-related proteins. Methods. Enzymol. 255, 65–82 (1995).
Philips, M. R. et al. Carboxyl methylation of Ras-related proteins during signal transduction in neutrophils. Science 259, 977–980 (1993).
Clarke, S., Vogel, J. P., Deschenes, R. J. & Stock, J. Posttranslational modification of the Ha-ras oncogene protein: evidence for a third class of protein carboxyl methyltransferases. Proc. Natl Acad. Sci. USA 85, 4643–4647 (1988).
Chen, Z. Q., Ulsh, L. S., DuBois, G. & Shih, T. Y. Posttranslational processing of p21 ras proteins involves palmitylation of the C-terminal tetrapeptide containing cysteine-186. J. Virol. 56, 607–612 (1985).
Coats, S. G., Booden, M. A. & Buss, J. E. Transient palmitoylation supports H-Ras membrane binding but only partial biological activity. Biochemistry 38, 12926–12934 (1999).
Kato, K., Der, C. J. & Buss, J. E. Prenoids and palmitate: lipids that control the biological activity of Ras proteins. Semin. Cancer Biol. 3, 179–188 (1992).
Hancock, J. F., Cadwallader, K., Paterson, H. & Marshall, C. J. A CAAX or a CAAL motif and a second signal are sufficient for plasma membrane targeting of ras proteins. EMBO J. 10, 4033–4039 (1991).
Jackson, J. H., Li, J. W., Buss, J. E., Der, C. J. & Cochrane, C. G. Polylysine domain of K-ras 4B protein is crucial for malignant transformation. Proc. Natl Acad. Sci. USA 91, 12730–12734 (1994).
Niv, H., Gutman, O., Kloog, Y. & Henis, Y. I. Activated K-Ras and H-Ras display different interactions with saturable nonraft sites at the surface of live cells. J. Cell Biol. 157, 865–872 (2002).
Prior, I. A. et al. GTP-dependent segregation of H-ras from lipid rafts is required for biological activity. Nature Cell. Biol. 3, 368–375 (2001).
Prior, I. A., Muncke, C., Parton, R. G. & Hancock, J. F. Direct visualization of Ras proteins in spatially distinct cell surface microdomains. J. Cell. Biol. 160, 165–170 (2003).
Prior, I. A. & Hancock, J. F. Compartmentalization of Ras proteins. J. Cell. Sci. 114, 1603–1608 (2001).
Pacold, M. E. et al. Crystal structure and functional analysis of Ras binding to its effector phosphoinositide 3-kinase γ. Cell 103, 931–943 (2000).
Corbett, K. D. & Alber, T. The many faces of Ras: recognition of small GTP-binding proteins. Trends Biochem. Sci. 26, 710–716 (2001).
Voice, J. K., Klemke, R. L., Le, A. & Jackson, J. H. Four human ras homologs differ in their abilities to activate Raf-1, induce transformation, and stimulate cell motility. J. Biol. Chem. 274, 17164–17170 (1999).
Kimmelman, A., Tolkacheva, T., Lorenzi, M. V., Osada, M. & Chan, A. M. Identification and characterization of R-ras3: a novel member of the RAS gene family with a non-ubiquitous pattern of tissue distribution. Oncogene 15, 2675–2685 (1997).
Quilliam, L. A. et al. M-Ras/R-Ras3, a transforming ras protein regulated by Sos1, GRF1, and p120 Ras GTPase-activating protein, interacts with the putative Ras effector AF6. J. Biol. Chem. 274, 23850–23857 (1999).
York, R. D. et al. Rap1 mediates sustained MAP kinase activation induced by nerve growth factor. Nature 392, 622–626 (1998).
Rodriguez-Viciana, P. et al. Phosphatidylinositol-3-OH kinase as a direct target of Ras. Nature 370, 527–532 (1994).
Yan, J., Roy, S., Apolloni, A., Lane, A. & Hancock, J. F. Ras isoforms vary in their ability to activate Raf-1 and phosphoinositide 3-kinase. J. Biol. Chem. 273, 24052–24056 (1998).
Kimmelman, A. C., Osada, M. & Chan, A. M. R-Ras3, a brain-specific Ras-related protein, activates Akt and promotes cell survival in PC12 cells. Oncogene 19, 2014–2022 (2000).
Rose, D. M., Cardarelli, P. M., Cobb, R. R. & Ginsberg, M. H. Soluble VCAM-1 binding to α4 integrins is cell-type specific and activation dependent and is disrupted during apoptosis in T cells. Blood 95, 602–609 (2000).
Acknowledgements
K.K., L.E.G., M.H. and F.L.C. are supported by postdoctoral fellowships from the University of California Tobacco-Related Disease Program, the American Cancer Society, the Danish Medical Research Council and the American Heart Association, respectively. Research in the Ginsberg laboratory is supported by the National Institutes of Health.
Author information
Authors and Affiliations
Corresponding author
Related links
Related links
DATABASES
Interpro
LocusLink
Swiss-Prot
Glossary
- AFFINITY
-
The strength of noncovalent chemical binding between two substances as measured by the dissociation constant of the complex.
- CAAX MOTIF
-
A carboxy-terminal tetrapeptide that is common to all Ras proteins and that directs a triplet of post-translational modifications.
- HYPERVARIABLE DOMAIN
-
The carboxy-terminal 25 amino acids of H-, N- and K-ras proteins in which sequence homology is less than 15% between any two isoforms compared with 90–100% over the amino-terminal sequences.
- DOMINANT-NEGATIVE
-
A defective protein that retains interaction capabilities and so distorts or competes with normal proteins.
- EOSINOPHIL
-
A type of white blood cell that has a bi-lobed nucleus and large cytoplasmic granules — containing hydrolytic enzymes — that stain readily with eosin.
- SWITCH REGIONS
-
The structures of Ras proteins resolved in either GDP- or GTP-bound form showed that the conformational change resulting from nucleotide exchange is mostly confined to the loop L2-β2 (according to Ras structural nomenclature) and the β3/α2 regions, which are termed switch I and II regions.
- MACROPHAGE
-
Any cell of the mononuclear phagocyte system that is characterized by its ability to phagocytose foreign particulate and colloidal material.
- OPSONIZATION
-
The process by which microorganisms or other particulate material are rendered more susceptible to phagocytosis by coating with opsonin.
- ADAPTOR PROTEIN
-
A protein that augments cellular responses by recruiting other proteins to a complex. Adaptor proteins usually contain several protein–protein interaction domains.
- SH3 DOMAIN
-
(Src-homology-3 domain). Protein sequences of about 50 amino acids that recognize and bind sequences that are rich in proline.
- EFFECTOR-LOOP-MUTANTS
-
Ras proteins with mutations in their effector-binding domains that abrogate their interactions with specific effectors.
- PLECKSTRIN HOMOLOGY (PH) DOMAIN
-
A sequence of 100 amino acids that is present in many signalling molecules and binds to lipid products of phosphatidylinositol 3-kinase. Pleckstrin is a protein of unknown function that was originally identified in platelets. It is a principal substrate of protein kinase C.
- AVIDITY
-
The strength of binding, usually of multiple ligand–receptor complexes in aggregation.
- MEGAKARYOCYTES
-
The source of blood platelets. The platelets are released by the megakaryocyte into the capillary sinuses. They are the largest cells in normal bone marrow.
- Fab FRAGMENT
-
The antigen-binding fragment of an immunoglobulin molecule. It is used when multimerization of antibodies caused by their Fc domains is not desirable.
Rights and permissions
About this article
Cite this article
Kinbara, K., Goldfinger, L., Hansen, M. et al. Ras GTPases: integrins' friends or foes?. Nat Rev Mol Cell Biol 4, 767–777 (2003). https://doi.org/10.1038/nrm1229
Issue Date:
DOI: https://doi.org/10.1038/nrm1229
This article is cited by
-
Role of microRNAs in regulation of doxorubicin and paclitaxel responses in lung tumor cells
Cell Division (2023)
-
ANXA6: a key molecular player in cancer progression and drug resistance
Discover Oncology (2023)
-
Connective tissue growth factor promotes chemotaxis of preosteoblasts through integrin α5 and Ras during tensile force-induced intramembranous osteogenesis
Scientific Reports (2021)
-
Reversible function of RapA with the C-terminus of RapC in Dictyostelium
Journal of Microbiology (2021)
-
Tissue engineering the cancer microenvironment—challenges and opportunities
Biophysical Reviews (2018)