Abstract
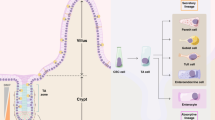
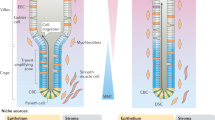
The colonic crypt is home to several multipotent stem cells. These stem cells reside in a niche at the base of the crypt, which controls their behavior and maintains the stem cell's homeostasis through a variety of signaling pathways and interactions. Several attempts have been made to define markers that can identify colonic stem cells, the most useful of which is Lgr5, a Wnt target gene. Although the crypt base contains several stem cells, each colonic crypt comprises a single clone of cells. Investigators have attempted to reconcile these apparently contradictory observations by conducting research into stem cell division. The propagation of stem-cell-acquired mutations through a crypt results in a monocryptal adenoma that, through crypt fission, develops into a microadenoma. Some early adenomas become polyclonal through an as yet unknown mechanism. The discovery of subpopulations of cancer cells that can initiate tumors when implanted into mice has renewed interest in the existence of cancer stem cells, especially with regard to their implications for the use of chemotherapy. Various potential markers of cancer stem cells have been investigated, particularly CD133, but the cancer stem cell theory still has some limitations.
Key Points
-
Several stem cells are thought to reside at the base of each colonic crypt, in a niche that maintains their homeostatic environment and regulates their activities
-
Colonic stem cells can divide either symmetrically (resulting in two daughter or two stem cells) or asymmetrically (resulting in one daughter and one stem cell)
-
The progeny of one stem cell will come to dominate the niche (niche succession) and eventually the entire crypt (monoclonal conversion), facilitating the spread of mutations within a crypt
-
Oncogenic mutations spread through the colonic epithelium (field cancerization) via crypt fission, during which crypts divide laterally; interactions between adjacent crypts might explain why many adenomas are polyclonal
-
Established tumors are thought to contain cancer stem cells, which could be responsible for the continued growth, invasion and metastasis of the tumor
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Cheng, H. & Leblond, C. P. Origin, differentiation and renewal of the four main epithelial cell types in the mouse small intestine. III. Entero-endocrine cells. Am. J. Anat. 141, 503–519 (1974).
Cheng, H. & Leblond, C. P. Origin, differentiation and renewal of the four main epithelial cell types in the mouse small intestine. V. Unitarian theory of the origin of the four epithelial cell types. Am. J. Anat. 141, 537–561 (1974).
Cheng, H. & Leblond, C. P. Origin, differentiation and renewal of the four main epithelial cell types in the mouse small intestine. I. Columnar cell. Am. J. Anat. 141, 461–479 (1974).
Stemple, D. L. & Anderson, D. J. Lineage diversification of the neural crest: in vitro investigations. Dev. Biol. 159, 12–23 (1993).
Beltrami, A. P. et al. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell 114, 763–776 (2003).
Mauro, A. Satellite cell of skeletal muscle fibers. J. Biophys. Biochem. Cytol. 9, 493–495 (1961).
Alonso, L. & Fuchs, E. Stem cells of the skin epithelium. Proc. Natl Acad. Sci. USA 100 (Suppl. 1), 11830–11835 (2003).
Bjerknes, M. & Cheng, H. The stem-cell zone of the small intestinal epithelium. V. Evidence for controls over orientation of boundaries between the stem-cell zone, proliferative zone, and the maturation zone. Am. J. Anat. 160, 105–112 (1981).
Bjerknes, M. & Cheng, H. The stem-cell zone of the small intestinal epithelium. I. Evidence from Paneth cells in the adult mouse. Am. J. Anat. 160, 51–63 (1981).
Bjerknes, M. & Cheng, H. The stem-cell zone of the small intestinal epithelium. IV. Effects of resecting 30% of the small intestine. Am. J. Anat. 160, 93–103 (1981).
Bjerknes, M. & Cheng, H. The stem-cell zone of the small intestinal epithelium. II. Evidence from Paneth cells in the newborn mouse. Am. J. Anat. 160, 65–75 (1981).
Bjerknes, M. & Cheng, H. The stem-cell zone of the small intestinal epithelium. III. Evidence from columnar, enteroendocrine, and mucous cells in the adult mouse. Am. J. Anat. 160, 77–91 (1981).
Forbes, S., Vig, P., Poulsom, R., Thomas, H. & Alison, M. Hepatic stem cells. J. Pathol. 197, 510–518 (2002).
Bonner-Weir, S. & Sharma, A. Pancreatic stem cells. J. Pathol. 197, 519–526 (2002).
Watt, F. M. & Hogan, B. L. Out of Eden: stem cells and their niches. Science 287, 1427–1430 (2000).
Luebeck, E. G. & Moolgavkar, S. H. Multistage carcinogenesis and the incidence of colorectal cancer. Proc. Natl Acad. Sci. USA 9, 15095–15100 (2002).
American Cancer Society. Colorectal Cancer Facts & Figures 2008–2010. Cancer Facts and Figures [online], (2010).
Potten, C. S. & Hendry, J. H. (Eds) Structure, function and proliferative organisation of the mammalian gut. In Radiation and Gut 1–31 (Elsevier, Amsterdam, 1995).
Bjerknes, M. & Cheng, H. Clonal analysis of mouse intestinal epithelial progenitors. Gastroenterology 116, 7–14 (1999).
Marshman, E., Booth, C. & Potten, C. The intestinal epithelial stem cell. Bioessays 24, 91–98 (2002).
Barker, N. et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 449, 1003–1007 (2007).
Kiger, A. A., Jones, D. L., Schulz, C., Rogers, M. B. & Fuller, M. T. Stem cell self-renewal specified by JAK–STAT activation in response to a support cell cue. Science 294, 2542–2545 (2001).
Tulina, N. & Matunis, E. Control of stem cell self-renewal in Drosophila spermatogenesis by JAK–STAT signaling. Science 294, 2546–2549 (2001).
Powell, D. W. et al. Myofibroblasts. II. Intestinal subepithelial myofibroblasts. Am. J. Physiol. 277 (Pt 1), C183–C201 (1999).
Okuno, T. et al. Interleukin-1β and tumour necrosis factor-α induce chemokine and matrix metalloproteinase gene expression in human colonic subepithelial myofibroblasts. Scand. J. Gastroenterol. 37, 317–324 (2002).
Mahida, Y. R. et al. Adult human colonic subepithelial myofibroblasts express extracellular matrix proteins and cyclooxygenase-1 and -2. Am. J. Physiol. 273 (Pt 1), G1341–G1348 (1997).
Korinek, V. et al. Depletion of epithelial stem-cell compartments in the small intestine of mice lacking Tcf-4. Nat. Genet. 19, 379–383 (1998).
Kuhnert, F. et al. Essential requirement for Wnt signaling in proliferation of adult small intestine and colon revealed by adenoviral expression of Dickkopf-1. Proc. Natl Acad. Sci. USA 101, 266–271 (2004).
Pinto, D., Gregorieff, A., Begthel, H. & Clevers, H. Canonical Wnt signals are essential for homeostasis of the intestinal epithelium. Genes Dev. 17, 1709–1713 (2003).
Batlle, E. et al. β-Catenin and TCF mediate cell positioning in the intestinal epithelium by controlling the expression of EphB/EphrinB. Cell 111, 251–263 (2002).
Kosinski, C. et al. Gene expression patterns of human colon tops and basal crypts and BMP antagonists as intestinal stem cell niche factors. Proc. Natl Acad. Sci. USA 104, 15418–15423 (2007).
Andoh, A., Bamba, S., Brittan, M., Fujiyama, Y. & Wright, N. A. Role of intestinal subepithelial myofibroblasts in inflammation and regenerative response in the gut. Pharmacol. Ther. 114, 94–106 (2007).
Crosnier, C., Stamataki, D. & Lewis, J. Organizing cell renewal in the intestine: stem cells, signals and combinatorial control. Nat. Rev. Genet. 7, 349–359 (2006).
He, X. C. et al. PTEN-deficient intestinal stem cells initiate intestinal polyposis. Nat. Genet. 39, 189–198 (2007).
He, X. C. et al. BMP signaling inhibits intestinal stem cell self-renewal through suppression of Wnt–β-catenin signaling. Nat. Genet. 36, 1117–1121 (2004).
Haramis, A. P. et al. De novo crypt formation and juvenile polyposis on BMP. Science 303, 1684–1686 (2004).
Hardwick, J. C., Kodach, L. L., Offerhaus, G. J. & van den Brink, G. R. Bone morphogenetic protein signalling in colorectal cancer. Nat. Rev. Cancer 8, 806–812 (2008).
van den Brink, G. R. et al. Indian Hedgehog is an antagonist of Wnt signaling in colonic epithelial cell differentiation. Nat. Genet. 36, 277–282 (2004).
Jensen, J. et al. Control of endodermal endocrine development by Hes-1. Nat. Genet. 24, 36–44 (2000).
van Es, J. H. et al. Notch/γ-secretase inhibition turns proliferative cells in intestinal crypts and adenomas into goblet cells. Nature 435, 959–963 (2005).
Yang, Q., Bermingham, N. A., Finegold, M. J. & Zoghbi, H. Y. Requirement of Math1 for secretory cell lineage commitment in the mouse intestine. Science 294, 2155–2158 (2001).
Madison, B. B. et al. Epithelial hedgehog signals pattern the intestinal crypt–villus axis. Development 132, 279–289 (2005).
Withers, H. R. & Elkind, M. M. Microcolony survival assay for cells of mouse intestinal mucosa exposed to radiation. Int. J. Radiat. Biol. Relat. Stud. Phys. Chem. Med. 17, 261–267 (1970).
Ponder, B. A. et al. Derivation of mouse intestinal crypts from single progenitor cells. Nature 313, 689–691 (1985).
Potten, C. S., Owen, G. & Booth, D. Intestinal stem cells protect their genome by selective segregation of template DNA strands. J. Cell Sci. 115 (Pt 11), 2381–2388 (2002).
Potten, C. S., Hume, W. J., Reid, P. & Cairns, J. The segregation of DNA in epithelial stem cells. Cell 15, 899–906 (1978).
Potten, C. S., Kellett, M., Rew, D. A. & Roberts, S. A. Proliferation in human gastrointestinal epithelium using bromodeoxyuridine in vivo: data for different sites, proximity to a tumour, and polyposis coli. Gut 33, 524–529 (1992).
Dekaney, C. M., Rodriguez, J. M., Graul, M. C. & Henning, S. J. Isolation and characterization of a putative intestinal stem cell fraction from mouse jejunum. Gastroenterology 129, 1567–1580 (2005).
Potten, C. S. et al. Identification of a putative intestinal stem cell and early lineage marker; musashi-1. Differentiation 71, 28–41 (2003).
Nishimura, S., Wakabayashi, N., Toyoda, K., Kashima, K. & Mitsufuji, S. Expression of Musashi-1 in human normal colon crypt cells: a possible stem cell marker of human colon epithelium. Dig. Dis. Sci. 48, 1523–1529 (2003).
May, R. et al. Identification of a novel putative gastrointestinal stem cell and adenoma stem cell marker, doublecortin and CaM kinase-like-1, following radiation injury and in adenomatous polyposis coli/multiple intestinal neoplasia mice. Stem Cells 26, 630–637 (2008).
Gerbe, F., Brulin, B., Makrini, L., Legraverend, C. & Jay, P. DCAMKL-1 expression identifies tuft cells rather than stem cells in the adult mouse intestinal epithelium. Gastroenterology 137, 2179–2180 (2009).
Barker, N. & Clevers, H. Leucine-rich repeat-containing G-protein-coupled receptors as markers of adult stem cells. Gastroenterology 138, 1681–1696 (2010).
Klaus, A. & Birchmeier, W. Wnt signalling and its impact on development and cancer. Nat. Rev. Cancer 8, 387–398 (2008).
van der Flier, L. G., Haegebarth, A., Stange, D. E. & van de Wetering, M. OLFM4 is a robust marker for stem cells in human intestine and marks a subset of colorectal cancer cells. Gastroenterology 137, 15–17 (2009).
Beà, B. et al. BMI-1 gene amplification and overexpression in hematological malignancies occur mainly in mantle cell lymphomas. Cancer Res. 61, 2409–2412 (2001).
Baylin, S. B., Herman, J. G., Graff, J. R., Vertino, P. M. & Issa, J. P. Alterations in DNA methylation: a fundamental aspect of neoplasia. Adv. Cancer Res. 72, 141–196 (1998).
Kim, H. K. et al. Circulating numbers of endothelial progenitor cells in patients with gastric and breast cancer. Cancer Lett. 198, 83–88 (2003).
Sangiorgi, E. & Capecchi, M. R. Bmi1 is expressed in vivo in intestinal stem cells. Nat. Genet. 40, 915–920 (2008).
Jass, J. R. & Roberton, A. M. Colorectal mucin histochemistry in health and disease: a critical review. Pathol. Int. 44, 487–504 (1994).
Fuller, C. E., Davies, R. P., Williams, G. T. & Williams, E. D. Crypt restricted heterogeneity of goblet cell mucus glycoprotein in histologically normal human colonic mucosa: a potential marker of somatic mutation. Br. J. Cancer 61, 382–384 (1990).
Novelli, M. R. et al. Polyclonal origin of colonic adenomas in an XO/XY patient with FAP. Science 272, 1187–1190 (1996).
Novelli, M. et al. X-inactivation patch size in human female tissue confounds the assessment of tumor clonality. Proc. Natl Acad. Sci. USA 100, 3311–3314 (2003).
Taylor, R. W. et al. Mitochondrial DNA mutations in human colonic crypt stem cells. J. Clin. Invest. 112, 1351–1360 (2003).
Shibata, D. Inferring human stem cell behaviour from epigenetic drift. J. Pathol. 217, 199–205 (2009).
Kim, K. M. & Shibata, D. Methylation reveals a niche: stem cell succession in human colon crypts. Oncogene 21, 5441–5449 (2002).
Shibata, D., Yatabe, Y. & Tavare, S. Investigating stem cells in human colon by using methylation patterns. Proc. Natl Acad. Sci. USA 98, 10839–10844 (2001).
Nicolas, P., Kim, K. M., Shibata, D. & Tavaré, S. The stem cell population of the human colon crypt: analysis via methylation patterns. PLoS Comput. Biol. 3, e28 (2007).
Snippert, H. J. et al. Intestinal crypt homeostasis results from neutral competition between symmetrically dividing Lgr5 stem cells. Cell 143, 134–144 (2010).
Cairns, J. Mutation selection and the natural history of cancer. Nature 255, 197–200 (1975).
Quyn, A. J. et al. Spindle orientation bias in gut epithelial stem cell compartments is lost in precancerous tissue. Cell Stem Cell 6, 175–181 (2010).
Falconer, E. et al. Identification of sister chromatids by DNA template strand sequences. Nature 463, 93–97 (2010).
Laken, S. J. et al. Analysis of masked mutations in familial adenomatous polyposis. Proc. Natl Acad. Sci. USA 96, 2322–2326 (1999).
Miyoshi, Y. et al. Somatic mutations of the APC gene in colorectal tumors: mutation cluster region in the APC gene. Hum. Mol. Genet. 1, 229–233 (1992).
Nakamura, S. & Kino, I. Morphogenesis of minute adenomas in familial polyposis coli. J. Natl Cancer Inst. 73, 41–49 (1984).
Woda, B. A., Forde, K. & Lane, N. A unicryptal colonic adenoma, the smallest colonic neoplasm yet observed in a non-polyposis individual. Am. J. Clin. Pathol. 68, 631–632 (1977).
Wasan, H. S. et al. APC in the regulation of intestinal crypt fission. J. Pathol. 185, 246–255 (1998).
Barker, N. et al. Crypt stem cells as the cells-of-origin of intestinal cancer. Nature 457, 608–611 (2009).
Roncucci, L., Medline, A. & Bruce, W. R. Classification of aberrant crypt foci and microadenomas in human colon. Cancer Epidemiol. Biomarkers Prev. 1, 57–60 (1991).
Levine, D. S. et al. Distribution of aneuploid cell populations in ulcerative colitis with dysplasia or cancer. Gastroenterology 101, 1198–1210 (1991).
Shih, I. M. et al. Top-down morphogenesis of colorectal tumors. Proc. Natl Acad. Sci. USA 98, 2640–2645 (2001).
Preston, S. L. et al. Bottom-up histogenesis of colorectal adenomas: origin in the monocryptal adenoma and initial expansion by crypt fission. Cancer Res. 63, 3819–3825 (2003).
Cheng, H., Bjerknes, M., Amar, J. & Gardiner, G. Crypt production in normal and diseased human colonic epithelium. Anat. Rec. 216, 44–48 (1986).
Maskens, A. P. & Dujardin-Loits, R. M. Kinetics of tissue proliferation in colorectal mucosa during post-natal growth. Cell Tissue Kinet. 14, 467–477 (1981).
Cairnie, A. B. & Millen, B. H. Fission of crypts in the small intestine of the irradiated mouse. Cell Tissue Kinet. 8, 189–196 (1975).
Wright, N. A. & Al-Nafussi, A. The kinetics of villus cell populations in the mouse small intestine. II. Studies on growth control after death of proliferative cells induced by cytosine arabinoside, with special reference to negative feedback mechanisms. Cell Tissue Kinet. 15, 611–621 (1982).
Greaves, L. C. et al. Mitochondrial DNA mutations are established in human colonic stem cells, and mutated clones expand by crypt fission. Proc. Natl Acad. Sci. USA 103, 714–719 (2006).
Chen, R. et al. The initiation of colon cancer in a chronic inflammatory setting. Carcinogenesis 26, 1513–1519 (2005).
Fialkow, P. J. Clonal origin of human tumors. Biochim. Biophys. Acta 458, 283–321 (1976).
Edwards, C. M. & Chapman, S. J. Biomechanical modelling of colorectal crypt budding and fission. Bull. Math. Biol. 69, 1927–1942 (2007).
Thliveris, A. T. et al. Polyclonality of familial murine adenomas: analyses of mouse chimeras with low tumor multiplicity suggest short-range interactions. Proc. Natl Acad. Sci. USA 102, 6960–6965 (2005).
Thirlwell, C. et al. Clonality assessment and clonal ordering of individual neoplastic crypts shows polyclonality of colorectal adenomas. Gastroenterology 138, 1441.e7–1454.e7 (2010).
Merritt, A. J., Gould, K. A. & Dove, W. F. Polyclonal structure of intestinal adenomas in ApcMin/+ mice with concomitant loss of Apc+ from all tumor lineages. Proc. Natl Acad. Sci. USA 94, 13927–13931 (1997).
Wu, M., Pastor-Pareja, J. C. & Xu, T. Interaction between RasV12 and scribbled clones induces tumour growth and invasion. Nature 463, 545–548 (2010).
Hamburger, A. W. & Salmon, S. E. Primary bioassay of human tumor stem cells. Science 197, 461–463 (1977).
Bonnet, D. & Dick, J. E. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat. Med. 3, 730–737 (1997).
Maw, M. A. et al. A frameshift mutation in prominin (mouse)-like 1 causes human retinal degeneration. Hum. Mol. Genet. 9, 27–34 (2000).
Miraglia, S. et al. A novel five-transmembrane hematopoietic stem cell antigen: isolation, characterization, and molecular cloning. Blood 90, 5013–5021 (1997).
Singh, S. K. et al. Identification of a cancer stem cell in human brain tumors. Cancer Res. 63, 5821–5828 (2003).
Singh, S. K. et al. Identification of human brain tumour initiating cells. Nature 432, 396–401 (2004).
Bussolati, B. et al. Isolation of renal progenitor cells from adult human kidney. Am. J. Pathol. 166, 545–555 (2005).
Collins, A. T., Berry, P. A., Hyde, C., Stower, M. J. & Maitland, N. J. Prospective identification of tumorigenic prostate cancer stem cells. Cancer Res. 65, 10946–10951 (2005).
Yin, S. et al. CD133 positive hepatocellular carcinoma cells possess high capacity for tumorigenicity. Int. J. Cancer 120, 1444–1450 (2007).
O'Brien, C. A., Pollett, A., Gallinger, S. & Dick, J. E. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature 445, 106–110 (2007).
Ricci-Vitiani, L. et al. Identification and expansion of human colon cancer initiating cells. Nature 445, 111–115 (2007).
Brabletz, T., Jung, A., Spaderna, S., Hlubek, F. & Kirchner, T. Opinion: migrating cancer stem cells—an integrated concept of malignant tumour progression. Nat. Rev. Cancer 5, 744–749 (2005).
Dalerba, P. et al. Phenotypic characterization of human colorectal cancer stem cells. Proc. Natl Acad. Sci. USA 104, 10158–10163 (2007).
Vermeulen, L. et al. Single-cell cloning of colon cancer stem cells reveals a multi-lineage differentiation capacity. Proc. Natl Acad. Sci. USA 105, 13427–13432 (2008).
Kirkland, S. C. Clonal origin of columnar, mucous, and endocrine cell lineages in human colorectal epithelium. Cancer 61, 1359–1363 (1988).
Schatton, T. et al. Identification of cells initiating human melanomas. Nature 451, 345–349 (2008).
Quintana, E. et al. Efficient tumour formation by single human melanoma cells. Nature 456, 593–598 (2008).
Dallas, N. A. et al. Chemoresistant colorectal cancer cells, the cancer stem cell phenotype, and increased sensitivity to insulin-like growth factor-I receptor inhibition. Cancer Res. 69, 1951–1957 (2009).
Author information
Authors and Affiliations
Contributions
S. S. Zeki researched data for and wrote the article. T. A. Graham and N. A. Wright made substantial contributions to discussions of the article content and to review and/or editing of the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
S. S. Zeki declares that he has received a research grant from the charitable organization CORE. The other authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Zeki, S., Graham, T. & Wright, N. Stem cells and their implications for colorectal cancer. Nat Rev Gastroenterol Hepatol 8, 90–100 (2011). https://doi.org/10.1038/nrgastro.2010.211
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrgastro.2010.211
This article is cited by
-
Identification and validation of fatty acid metabolism-related lncRNA signatures as a novel prognostic model for clear cell renal cell carcinoma
Scientific Reports (2023)
-
Prospects of plant-derived exosome-like nanocarriers in oncology and tissue engineering
Human Cell (2023)
-
CircRNAs: emerging factors for regulating glucose metabolism in colorectal cancer
Clinical and Translational Oncology (2023)
-
Nonviral mcDNA-mediated bispecific CAR T cells kill tumor cells in an experimental mouse model of hepatocellular carcinoma
BMC Cancer (2022)
-
Significant co-expression of putative cancer stem cell markers, EpCAM and CD166, correlates with tumor stage and invasive behavior in colorectal cancer
World Journal of Surgical Oncology (2022)