Key Points
-
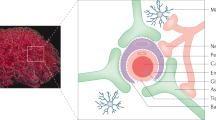
The brains of all vertebrates are protected from substances in the blood by the blood–brain barrier (BBB). Although the BBB maintains brain function under normal conditions, it excludes the penetration into the brain of >98% of small-molecule drugs and virtually 100% of large-molecule drugs or gene medicines.
-
One solution to the BBB drug- and gene-delivery problem is chimeric peptide technology. In the formulation of a chimeric peptide, a drug that is normally not transported across the BBB is conjugated or fused to a BBB transport vector. The vector is an endogenous peptide or peptidomimetic monoclonal antibody that undergoes receptor-mediated transcytosis (RMT) through the BBB in vivo.
-
BBB transport vectors act as molecular Trojan Horses, which ferry the drug or gene across the BBB on the endogenous-peptide RMT system. The drug or gene can be attached to the Trojan Horse by either genetic engineering or avidin–biotin technology.
-
Neurotrophins are strongly neuroprotective if injected directly into the brain immediately after brain ischaemia, but are inactive following intravenous administration in the absence of BBB disruption because these large-molecule drugs do not cross the BBB. Neurotrophin chimeric peptides cause complete neuroprotection in the hippocampus after transient global brain ischaemia, and a 70% reduction in stroke volume in regional ischaemia after delayed intravenous administration, because these molecules have been enabled to cross the BBB.
-
Peptide radiopharmaceuticals could allow the early detection of brain diseases, such as brain cancer or Alzheimer's disease, should these molecules be made transportable through the BBB. The epidermal growth factor (EGF) receptor is overexpressed in brain cancer. An experimental human brain cancer could not be imaged with an EGF peptide radiopharmaceutical, because the peptide does not cross the BBB. However, the brain cancer was imaged with an EGF chimeric peptide that was able to cross the blood–tumour barrier after intravenous administration.
-
The imaging of gene expression in vivo is possible with antisense radiopharmaceuticals if these highly charged agents are able to cross both the BBB and the brain cell membrane in vivo. The conjugation of a sequence-specific antisense radiopharmaceutical to a molecular Trojan Horse has been shown to enable the imaging of a target gene in the brain in vivo after intravenous administration.
-
Widespread expression of a therapeutic gene throughout the central nervous system is required to treat many intractable neurological diseases. This is now possible with brain gene-targeting technology, using a combination of molecular Trojan Horses, non-viral gene medicines and tissue-specific gene promoters.
-
The BBB is the rate-limiting factor in the translation of progress in the molecular neurosciences into clinically effective neurotherapeutics.
Abstract
Getting drugs and genes into the brain is a tall order. This is because the presence of the blood–brain barrier prevents many molecules from crossing into the brain. Overcoming this problem will have a profound effect on the treatment of many neurological disorders, allowing larger water-soluble molecules to pass into the brain. Transport vectors, such as endogenous peptides, modified proteins or peptidomimetic monoclonal antibodies, are one way of tricking the brain into allowing these molecules to pass. This article will review such molecular Trojan Horses, and the progress that has been made in the delivery of drugs and genes to the brain.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Pardridge, W. M. Brain Drug Targeting: the Future of Brain Drug Development (Cambridge Univ. Press, Cambridge, 2001).
Cserr, H. F., Fenstermacher, J. D. & Rall, D. P. Comparative aspects of brain barrier systems for nonelectrolytes. Am. J. Physiol. 234, R52–R60 (1978).
Mollgard, K. & Saunders, N. R. Complex tight junctions of epithelial and of endothelial cells in early fetal brain. J. Neurocytol. 4, 453–468 (1975).
Brightman, M. W. Morphology of blood–brain interfaces. Exp. Eye Res. 25 (Suppl.), 1–25 (1977).This paper reviews the classical electron-microscopic histochemistry that showed that the anatomical basis of the BBB is the capillary endothelial-cell tight junction.
Pardridge, W. M. Peptide Drug Delivery to the Brain (Raven, New York, 1991).
Pardridge, W. M., Yang, J., Buciak, J. & Tourtellotte, W. W. Human brain microvascular DR antigen. J. Neurosci. Res. 23, 337–341 (1989).
Paulson, O. B. & Newman, E. A. Does the release of potassium from astrocyte endfeet regulate cerebral blood flow? Science 237, 896–898 (1987).
Pardridge, W. M. CNS drug design based on principles of blood–brain barrier transport. J. Neurochem. 70, 1781–1792 (1998).
Zhang, Y. & Pardridge, W. M. Mediated efflux of IgG molecules from brain to blood across the blood–brain barrier. J. Neuroimmunol. 114, 168–172 (2001).
Skarlatos, S., Yoshikawa, T. & Pardridge, W. M. Transport of [125I]-transferrin through the rat blood–brain barrier in vivo. Brain Res. 683, 164–171 (1995).
Zhang, Y. & Pardridge, W. M. Rapid transferrin efflux from brain to blood across the blood–brain barrier. J. Neurochem. 76, 1597–1600 (2001).
Green, N. M. Avidin. Adv. Prot. Chem. 29, 85–133 (1975).
Belayev, L., Busto, R., Zhao, W. & Ginsberg, M. D. Quantitative evaluation of blood–brain barrier permeability following middle cerebral artery occlusion in rats. Brain Res. 739, 88–96 (1996). This investigation shows that the BBB is intact in the first 4–6 hours after experimental regional ischaemia, similar to other forms of acute brain injury. Neuroprotective agents will therefore not be effective unless they can cross the BBB.
Albayrak, S., Zhao, Q., Siesjo, B. K. & Smith, M. L. Effect of transient focal ischemia on blood–brain barrier permeability in the rat: correlation to cell injury. Acta Neuropathol. (Berl.) 94, 158–163 (1997).
Kaplan, B. et al. Temporal thresholds for neocortical infarction in rats subjected to reversible focal cerebral ischemia. Stroke 22, 1032–1039 (1991).
Wu, D. & Pardridge, W. M. Neuroprotection with non-invasive neurotrophin delivery to brain. Proc. Natl Acad. Sci. USA 96, 254–259 (1999).
Zhang, Y. & Pardridge, W. M. Conjugation of brain-derived neurotrophic factor to a blood–brain barrier drug targeting system enables neuroprotection in regional brain ischemia following intravenous injection of the neurotrophin. Brain Res. 889, 49–56 (2001).
Zhang, Y. & Pardridge, W. M. Neuroprotection in transient focal brain ischemia following delayed, intravenous administration of BDNF conjugated to a blood–brain barrier drug targeting system. Stroke 32, 1378–1384 (2001).
Hefti, F. Pharmacology of neurotrophic factors. Annu. Rev. Pharmacol. Toxicol. 37, 239–267 (1997).
Beck, T., Lindholm, D., Castren, E. & Wree, A. Brain-derived neurotrophic factor protects against ischemic cell damage in rat hippocampus. J. Cereb. Blood Flow Metab. 14, 689–692 (1994).
Yamashita, K., Wiessner, C., Lindholm, D., Thoenen, H. & Hossmann, K. Post-occlusion treatment with BDNF reduces infarct size in a model of permanent occlusion of the middle cerebral artery in rat. Metab. Brain Dis. 12, 271–280 (1997).
Wong, A. J. et al. Increased expression of the epidermal growth factor receptor gene in malignant gliomas is invariably associated with gene amplification. Proc. Natl Acad. Sci. USA 84, 6899–6903 (1987).
Nishikawa, R. et al. A mutant epidermal growth factor receptor common in human glioma confers enhanced tumorigenicity. Proc. Natl Acad. Sci. USA 91, 7727–7731 (1994).
Kurihara, A., Deguchi, Y. & Pardridge, W. M. Epidermal growth factor radiopharmaceuticals: 111In chelation, conjugation to a blood–brain barrier delivery vector via a biotin–polyethylene linker, pharmacokinetics, and in vivo imaging of experimental brain tumors. Bioconjug. Chem. 10, 502–511 (1999).
Kurihara, A. & Pardridge, W. M. Imaging brain tumors by targeting peptide radiopharmaceuticals through the blood–brain barrier. Cancer Res. 59, 6159–6163 (1999).
Deguchi, Y., Kurihara, A. & Pardridge, W. M. Retention of biologic activity of human epidermal growth factor following conjugation to a blood–brain barrier drug delivery vector via an extended polyethyleneglycol linker. Bioconjug. Chem. 10, 32–37 (1999).
Morris, J. C. et al. Cerebral amyloid deposition and diffuse plaques in 'normal' aging: evidence for presymptomatic and very mild Alzheimer's disease. Neurology 47, 707–719 (1996).
Saito, Y., Buciak, J., Yang, J. & Pardridge, W. M. Vector-mediated delivery of [125I]-labeled β-amyloid peptide Aβ1–40 through the blood–brain barrier and binding to Alzheimer's Disease amyloid of the Aβ1–40/vector complex. Proc. Natl Acad. Sci. USA 92, 10227–10231 (1995).
Kurihara, A. & Pardridge, W. M. Aβ1–40 radiopharmaceuticals for brain amyloid imaging, (111)In chelation, conjugation to poly(ethlyeneglycol)–biotin linkers, and autoradiography with Alzheimer's disease brain sections. Bioconjug. Chem. 11, 380–386 (2000).
Lee, H. J., Zhang, Y., Zhu, C., Duff, K. & Pardridge, W. M. Imaging brain amyloid of Alzheimer's disease in vivo in transgenic mice with an Aβ peptide radiopharmaceutical. J. Cereb. Blood Flow Metab. 22, 222–231 (2001).
Brown, D. et al. Effect of phosphorothioate of oligodeoxynucleotides of specific protein binding. J. Biol. Chem. 269, 26801–26805 (1994).
Cossum, P. A. et al. Disposition of the 14C-labeled phosphorothioate oligonucleotide ISIS 2015 after intravenous administration to rats. J. Pharmacol. Exp. Ther. 267, 1181–1190 (1993).
Wu, D., Boado, R. J. & Pardridge, W. M. Pharmacokinetics and blood–brain barrier transport of [3H]-biotinylated phosphorothioate oligodeoxynucleotide conjugated to a vector-mediated drug delivery system. J. Pharmacol. Exp. Ther. 276, 206–211 (1996).
Pardridge, W. M., Boado, R. J. & Kang, Y.-S. Vector-mediated delivery of a polyamide ('peptide') nucleic acid analogue through the blood–brain barrier in vivo. Proc. Natl Acad. Sci. USA 92, 5592–5596 (1995).
Hanvey, J. C. et al. Antisense and antigene properties of peptide nucleic acids. Science 258, 1481–1486 (1992).
Shi, N., Boado, R. J. & Pardridge, W. M. Antisense imaging of gene expression in the brain in vivo. Proc. Natl Acad. Sci. USA 97, 14709–14714 (2000).
Kajiwara, K. et al. Humoral immune responses to adenovirus vectors in the brain. J. Neuroimmunol. 103, 8–15 (2000).
Herrlinger, U. et al. Pre-existing herpes simplex virus 1 (HSV-1) immunity decreases but does not abolish, gene transfer to experimental brain tumors by a HSV-1 vector. Gene Ther. 5, 809–819 (1998).
Driesse, M. J. et al. Intracerebral injection of adenovirus harboring the HSVtk gene combined with ganciclovir administration: toxicity study in nonhuman primates. Gene Ther. 5, 1122–1129 (1998).
Lawrence, M. S. et al. Inflammatory responses and their impact on β-galactosidase transgene expression following adenovirus vector delivery to the primate caudate nucleus. Gene Ther. 6, 1368–1379 (1999).A single injection of adenovirus into the primate brain causes local inflammation that leads to demyelination.
Kramm, C. M. et al. Herpes vector-mediated delivery of marker genes to disseminated central nervous system tumors. Hum Gene Ther 7, 291–300 (1996).
McMenamin, M. M. et al. A γ34.5 mutant of herpes simplex 1 causes severe inflammation in the brain. Neuroscience 83, 1225–1237 (1998).Even replication-deficient strains of the herpes simplex virus are toxic to the brain, causing local inflammation, increased antigen-presenting cells and perivascular lymphocyte cuffing.
Hofland, H. E. J. et al. In vivo gene transfer by intravenous administration of stable cationic lipid/DNA complex. Pharm. Res. 14, 742–749 (1997).
Plank, C., Tang, M. X., Wolfe, A. R. & Szoka, F. C. Jr. Branched cationic peptides for gene delivery: role of type and number of cationic residues in formation and in vitro activity of DNA polyplexes. Hum. Gene Ther. 10, 319–332 (1999).
Osaka, G. et al. Pharmacokinetics, tissue distribution, and expression efficiency of plasmid [33P]DNA following intravenous administration of DNA/cationic lipid complexes in mice: use of a novel radionuclide approach. J. Pharm. Sci. 85, 612–618 (1996).
Shi, N. & Pardridge, W. M. Non-invasive gene targeting to the brain. Proc. Natl Acad. Sci. USA 97, 7567–7572 (2000).
Shi, N., Boado, R. J. & Pardridge, W. M. Receptor-mediated gene targeting to tissues in the rat in vivo. Pharm. Res. 18, 1091–1095 (2001).
Cruz, M. T., Simoes, S., Pires, P. P. C., Nir, S. & Lima, M. C. Kinetic analysis of the initial steps involved in lipoplex–cell interactions: effect of various factors that influence transfection activity. Biochim. Biophys. Acta 1510, 136–151 (2001).
Zhang, Y., Lee, H. J., Boado, R. J. & Pardridge, W. M. Receptor-mediated delivery of an antisense gene to human brain cancer cells. J. Gene Med. (in the press).
Papahadjopoulos, D. et al. Sterically stabilized liposomes: improvements in pharmacokinetics and antitumor therapeutic efficacy. Proc. Natl Acad. Sci. USA 88, 11460–11464 (1991).This classical study shows that the conjugation of PEG to the surface of liposomes greatly prolongs the blood residence time and optimizes the pharmacokinetics.
Shi, N., Zhang, Y., Boado, R. J., Zhu, C. & Pardridge, W. M. Brain-specific expression of an exogenous gene following intravenous administration. Proc. Natl Acad. Sci. USA 98, 12754–12759 (2001).
Lee, H. J., Engelhardt, B., Lesley, J., Bickel, U. & Pardridge, W. M. Targeting rat anti-mouse transferrin receptor monoclonal antibodies through the blood–brain barrier. J. Pharmacol. Exp. Ther. 292, 1048–1052 (2000).
Pardridge, W. M., Kang, Y.-S., Buciak, J. L. & Yang, J. Human insulin receptor monoclonal antibody undergoes high affinity binding to human brain capillaries in vitro and rapid transcytosis through the blood–brain barrier in vivo in the primate. Pharm. Res. 12, 807–816 (1995).
Coloma, M. J. et al. Transport across the primate blood–brain barrier of a genetically engineered chimeric monoclonal antibody to the human insulin receptor. Pharm. Res. 17, 266–274 (2000). This study describes the genetic engineering of a brain transport vector and shows targeting to the primate brain in vivo . The genetically engineered vector could be used to target drugs or genes to the human brain.
Pardridge et al. Blood–brain barrier: interface between internal medicine and the brain. Ann. Intern. Med. 105, 82–95 (1986).
Duvernoy, H., Delon, S. & Vannson, J. L. The vascularization of the human cerebellar cortex. Brain Res. Bull. 11, 419–480 (1983).
Cornford, E. M., Hyman, S., Cornford, M. E. & Caron, M. J. GLUT1 glucose transporter activity in human brain injury. J. Neurotrauma 13, 523–536 (1996).
Brownlees, J. & Williams, C. H. Peptidases, peptides, and the mammalian blood–brain barrier. J. Neurochem. 60, 793–803 (1993).
Tsuji, A. & Tamai, I. Carrier-mediated or specialized transport of drugs across the blood–brain barrier. Adv. Drug Deliv. Rev. 36, 277–290 (1999).
Takasawa, K., Terasaki, T., Suzuki, H. & Sugiyama, Y. In vivo evidence for carrier-mediated efflux transport of 3′-azido-3′-deoxythymidine and 2′,3′-dideoxyinosine across the blood–brain barrier via a probenecid-sensitive transport system. J. Pharmacol. Exp. Ther. 281, 369–375 (1997).
Pardridge, W. M. Receptor-mediated peptide transport through the blood–brain barrier. Endocr. Rev. 7, 314–330 (1986).
Krewson, C. E., Klarman, M. L. & Saltzman, W. M. Distribution of nerve growth factor following direct delivery to brain interstitium. Brain Res. 680, 196–206 (1995).
Yan, Q. et al. Distribution of intracerebral ventricularly administered neurotrophins in rat brain and its correlation with Trk receptor expression. Exp. Neurol. 127, 23–36 (1994).
Author information
Authors and Affiliations
Related links
Related links
DATABASES
FURTHER INFORMATION
Glossary
- PEPTIDOMIMETIC
-
A molecule that binds to a blood–brain barrier (BBB) receptor and initiates receptor-mediated transcytosis (RMT) across the BBB. A peptidomimetic monoclonal antibody binds to an extracellular projecting epitope on the receptor to trigger RMT in a manner similar to the endogenous ligand for the receptor.
- ENDOTHELIUM
-
Consists of cells of mesenchymal origin that comprise the innermost cellular lining of the capillary.
- TRANSCYTOSIS
-
Transport across the endothelial barrier by movement through the endothelial cytoplasm (the transcellular pathway), in contrast to transport across the endothelial barrier by movement through the inter-endothelial junctional spaces (the paracellular pathway).
- PINOCYTOSIS
-
Transcytosis across the endothelial cell through endosomal vesicles and/or a tubulovesicular network within the endothelial cell.
- MICROVASCULATURE
-
The capillary network that perfuses the brain and delivers to the brain circulating nutrients and oxygen.
- PERICYTE
-
A multifunctional cell that shares the basement membrane of the microvasculature with the endothelium, and sits on the brain side of the capillary.
- EXOFACIAL
-
Part of a plasma-membrane receptor that projects outwards into the extracellular space.
- NEUROTROPHIN
-
A protein produced within the brain that modulates neuronal function and structure.
- CEREBRAL INFARCTION
-
Loss of brain tissue subsequent to the transient or permanent loss of circulation and/or oxygen delivery to that region of the brain; also known as a stroke.
- XENOBIOTIC
-
A drug or naturally occuring alkaloid that has pharmacological properties.
- EPENDYMAL SURFACE
-
The surface of the brain that is lined by the ependymal epithelium, which is in contact with the cerebrospinal fluid.
- AMYLOID
-
A silk-like protein aggregate that consists of a peptide that has a high degree of β-pleated-sheet secondary structure, which enables the aggregation of the peptide into plaques that deposit in the extracellular space of the brain.
- RETICULO-ENDOTHELIAL
-
A system of phagocytic cells that line the microvasculature of the liver and spleen, and which remove foreign substances or particles from the blood.
Rights and permissions
About this article
Cite this article
Pardridge, W. Drug and gene targeting to the brain with molecular trojan horses. Nat Rev Drug Discov 1, 131–139 (2002). https://doi.org/10.1038/nrd725
Issue Date:
DOI: https://doi.org/10.1038/nrd725
This article is cited by
-
Pericyte-derived exosomal miR-210 improves mitochondrial function and inhibits lipid peroxidation in vascular endothelial cells after traumatic spinal cord injury by activating JAK1/STAT3 signaling pathway
Journal of Nanobiotechnology (2023)
-
Nucleic acid drug vectors for diagnosis and treatment of brain diseases
Signal Transduction and Targeted Therapy (2023)
-
The blood-brain barrier in aging and neurodegeneration
Molecular Psychiatry (2022)
-
A review of glucoregulatory hormones potentially applicable to the treatment of Alzheimer’s disease: mechanism and brain delivery
Journal of Pharmaceutical Investigation (2022)
-
New Drug Delivery Systems Developed for Brain Targeting
Drugs (2022)