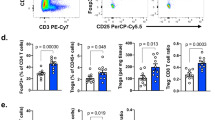
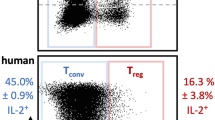
Key Points
-
Dominant tolerance is executed by a committed lineage of CD4+CD25+Foxp3+ regulatory T (TReg) cells.
-
The fact that TReg cells are essential mediators of immune homeostasis has led to the quest for novel therapeutic strategies for targeting these cells in various diseases.
-
In autoimmunity, the induction of specific TReg cells could permit modulation of the immune response for clinical benefit while limiting the effect of long-term general immune suppression.
-
Strategies to promote TReg cell generation include subimmunogenic T cell receptor stimulation using strongly agonistic variants of self-antigens, transforming growth factor-β, inhibitors of the mammalian target of rapamycin (mTOR) pathway as well as microRNAs.
-
Approaches to expand TReg cells include the application of cytokines such as interleukin-2 (IL-2), whereas the manipulation of TReg cell survival and stability can be achieved using phosphoinositide 3-kinase (PI3K)–AKT–mTOR inhibitors or DNA methyltransferase inhibitors.
-
In cancer, the development of agents that specifically inhibit TReg cell function will permit new approaches for anticancer immunotherapy.
-
Blockade of TReg cell expansion can be achieved using tyrosine kinase inhibitors such as imatinib, which can also support the inhibition of the suppressive function of TReg cells.
-
TReg cell-mediated immune suppression can be limited by low doses of cyclophosphamide or by cytotoxic T lymphocyte antigen 4 (CTLA4)-blocking antibodies such as ipilimumab.
-
Combinatorial approaches of antigen-specific and nonspecific strategies, along with an improved understanding of the underlying molecular mechanisms of tolerance, are likely to be required to deal with the complexity of the immune system.
Abstract
Forkhead box P3 (FOXP3)-expressing regulatory T (TReg) cells have a pivotal role in the regulation of immune responses and in the maintenance of immunological self-tolerance. These cells have emerged as attractive targets for strategies that allow the steering of immune responses in desired directions — arming the immune system to destroy infected cells and cancer cells or downregulating it to limit tissue destruction in autoimmunity. Efforts to understand the generation, activation and function of TReg cells should permit the development of therapeutics for reprogramming the immune system. In this Review, we discuss insights into the generation of TReg cells, their involvement in disease and the molecular basis of the dominant tolerance exerted by FOXP3+ TReg cells that could permit their safe and specific manipulation in humans.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Burnet, F. M. The Clonal Selection Theory (Cambridge Press; 1959).
Kappler, J. W., Roehm, N. & Marrack, P. T cell tolerance by clonal elimination in the thymus. Cell 49, 273–280 (1987).
Kisielow, P., Bluthmann, H., Staerz, U.D., Steinmetz, M. & von Boehmer, H. Tolerance in T-cell receptor transgenic mice involves deletion of nonmature CD4+8+ thymocytes. Nature 333, 742–746 (1988).
Lederberg, J. Genes and antibodies. Science 129, 1649–1653 (1959).
Fontenot, J. D., Gavin, M. A. & Rudensky, A. Y. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nature Immunol. 4, 330–336 (2003).
Khattri, R., Cox, T., Yasayko, S. A. & Ramsdell, F. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nature Immunol. 4, 337–342 (2003).
Modigliani, Y., Bandeira, A. & Coutinho, A. A model for developmentally acquired thymus-dependent tolerance to central and peripheral antigens. Immunol. Rev. 149, 155–174 (1996).
Modigliani, Y. et al. Lymphocytes selected in allogeneic thymic epithelium mediate dominant tolerance toward tissue grafts of the thymic epithelium haplotype. Proc. Natl Acad. Sci. USA 92, 7555–7559 (1995).
Chen, Y., Kuchroo, V. K., Inobe, J., Hafler, D. A. & Weiner, H. L. Regulatory T cell clones induced by oral tolerance: suppression of autoimmune encephalomyelitis. Science 265, 1237–1240 (1994).
Miller, A., Lider, O. & Weiner, H. L. Antigen-driven bystander suppression after oral administration of antigens. J. Exp. Med. 174, 791–798 (1991).
Verginis, P., McLaughlin, K. A., Wucherpfennig, K. W., von Boehmer, H. & Apostolou, I. Induction of antigen-specific regulatory T cells in wild-type mice: visualization and targets of suppression. Proc. Natl Acad. Sci. USA 105, 3479–3484 (2008).
Asano, M., Toda, M., Sakaguchi, N. & Sakaguchi, S. Autoimmune disease as a consequence of developmental abnormality of a T cell subpopulation. J. Exp. Med. 184, 387–396 (1996).
Kim, J. M., Rasmussen, J. P. & Rudensky, A. Y. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nature Immunol. 8, 191–197 (2007).
Lahl, K. et al. Selective depletion of Foxp3+ regulatory T cells induces a scurfy-like disease. J. Exp. Med. 204, 57–63 (2007).
Ochs, H. D., Ziegler, S. F. & Torgerson, T. R. FOXP3 acts as a rheostat of the immune response. Immunol. Rev. 203, 156–164 (2005).
Buckner, J. H. Mechanisms of impaired regulation by CD4+CD25+FOXP3+ regulatory T cells in human autoimmune diseases. Nature Rev. Immunol. 10, 849–859 (2010).
Clough, L. E. et al. Release from regulatory T cell-mediated suppression during the onset of tissue-specific autoimmunity is associated with elevated IL-21. J. Immunol. 180, 5393–5401 (2008).
Korn, T. et al. Myelin-specific regulatory T cells accumulate in the CNS but fail to control autoimmune inflammation. Nature Med. 13, 423–431 (2007).
Fairchild, P. J., Wildgoose, R., Atherton, E., Webb, S. & Wraith, D. C. An autoantigenic T cell epitope forms unstable complexes with class II MHC: a novel route for escape from tolerance induction. Int. Immunol. 5, 1151–1158 (1993).
Garcia, K. C., Teyton, L. & Wilson, I. A. Structural basis of T cell recognition. Annu. Rev. Immunol. 17, 369–397 (1999).
Hahn, M., Nicholson, M. J., Pyrdol, J. & Wucherpfennig, K. W. Unconventional topology of self peptide-major histocompatibility complex binding by a human autoimmune T cell receptor. Nature Immunol. 6, 490–496 (2005).
Liu, G. Y. et al. Low avidity recognition of self-antigen by T cells permits escape from central tolerance. Immunity 3, 407–415 (1995).
Stadinski, B. D. et al. Diabetogenic T cells recognize insulin bound to IAg7 in an unexpected, weakly binding register. Proc. Natl Acad. Sci. USA 107, 10978–10983 (2010). This paper supports the concept that the relevant insulin B-chain epitope is presented by MHCII I-Ag7 molecules in an unfavoured binding register, which is associated with weak agonistic activity of the peptide–MHC complex.
Wucherpfennig, K. W. & Sethi, D. T cell receptor recognition of self and foreign antigens in the induction of autoimmunity. Semin. Immunol. 23, 84–91 (2011).
Grindebacke, H. et al. Defective suppression of Th2 cytokines by CD4CD25 regulatory T cells in birch allergics during birch pollen season. Clin. Exp. Allergy 34, 1364–1372 (2004).
Ling, E. M. et al. Relation of CD4+CD25+ regulatory T-cell suppression of allergen-driven T-cell activation to atopic status and expression of allergic disease. Lancet 363, 608–615 (2004).
Bellinghausen, I., Klostermann, B., Knop, J. & Saloga, J. Human CD4+CD25+ T cells derived from the majority of atopic donors are able to suppress TH1 and TH2 cytokine production. J. Allergy Clin. Immunol. 111, 862–868 (2003).
Cavani, A. et al. Human CD25+ regulatory T cells maintain immune tolerance to nickel in healthy, nonallergic individuals. J. Immunol. 171, 5760–5768 (2003).
Taams, L. S. et al. Antigen-specific T cell suppression by human CD4+CD25+ regulatory T cells. Eur. J. Immunol. 32, 1621–1630 (2002).
Zou, W. Regulatory T cells, tumour immunity and immunotherapy. Nature Rev. Immunol. 6, 295–307 (2006).
Viguier, M. et al. Foxp3 expressing CD4+CD25high regulatory T cells are overrepresented in human metastatic melanoma lymph nodes and inhibit the function of infiltrating T cells. J. Immunol. 173, 1444–1453.
Wolf, A. M. et al. Increase of regulatory T cells in the peripheral blood of cancer patients. Clin. Cancer Res. 9, 606–612 (2003).
Woo, E. Y. et al. Cutting edge: regulatory T cells from lung cancer patients directly inhibit autologous T cell proliferation. J. Immunol. 168, 4272–4276 (2002).
Galluzzi, L., Senovilla, L., Zitvogel, L. & Kroemer, G. The secret ally: immunostimulation by anticancer drugs. Nature Rev. Drug Discov. 11, 215–233 (2012).
Lesterhuis, W. J., Haanen, J. B. & Punt, C. J. Cancer immunotherapy — revisited. Nature Rev. Drug Discov. 10, 591–600 (2011).
Zitvogel, L. et al. The anticancer immune response: indispensable for therapeutic success? J. Clin. Invest. 118, 1991–2001 (2008).
Roncarolo, M. G. & Battaglia, M. Regulatory T-cell immunotherapy for tolerance to self antigens and alloantigens in humans. Nature Rev. Immunology 7, 585–598 (2007).
Li, M. O. & Flavell, R. A. TGF-β: a master of all T cell trades. Cell 134, 392–404 (2008).
Polansky, J. K. et al. DNA methylation controls Foxp3 gene expression. Eur. J. Immunol. 38, 1654–1663 (2008).
Moustakas, A., Souchelnytskyi, S. & Heldin, C. H. Smad regulation in TGF-β signal transduction. J. Cell Sci. 114, 4359–4369 (2001).
Zheng, Y. et al. Role of conserved non-coding DNA elements in the Foxp3 gene in regulatory T-cell fate. Nature 463, 808–812 (2010).
Samstein, R. M., Josefowicz, S. Z., Arvey, A., Treuting, P. M. & Rudensky, A. Y. Extrathymic generation of regulatory T cells in placental mammals mitigates maternal-fetal conflict. Cell 150, 29–38 (2012). This paper elucidates a crucial mechanism during evolution in placental animals in order to enforce maternofetal tolerance.
Ruan, Q. et al. Development of Foxp3+ regulatory T cells is driven by the c-Rel enhanceosome. Immunity 31, 932–940 (2009).
Schlenner, S. M., Weigmann, B., Ruan, Q., Chen, Y. & von Boehmer, H. Smad3 binding to the foxp3 enhancer is dispensable for the development of regulatory T cells with the exception of the gut. J. Exp. Med. 209, 1529–1535 (2012). This paper makes use of Foxp3 -mutant mice, in which binding of SMAD3 to CNS1 was abrogated, to show that in vivo SMAD3 binding to CNS1 is required for T Reg cell generation only in the gut.
Tran, D. Q., Ramsey, H. & Shevach, E. M. Induction of FOXP3 expression in naive human CD4+FOXP3 T cells by T-cell receptor stimulation is transforming growth factor-β dependent but does not confer a regulatory phenotype. Blood 110, 2983–2990 (2007).
Almeida, A. R., Legrand, N., Papiernik, M. & Freitas, A. A. Homeostasis of peripheral CD4+ T cells: IL-2R α and IL-2 shape a population of regulatory cells that controls CD4+ T cell numbers. J. Immunol. 169, 4850–4860 (2002).
Malek, T. R., Yu, A., Vincek, V., Scibelli, P. & Kong, L. CD4 regulatory T cells prevent lethal autoimmunity in IL-2Rβ-deficient mice. Implications for the nonredundant function of IL-2. Immunity 17, 167–178 (2002).
Boyman, O., Kovar, M., Rubinstein, M. P., Surh, C. D. & Sprent, J. Selective stimulation of T cell subsets with antibody-cytokine immune complexes 6. Science 311, 1924–1927 (2006).
Daniel, C., Wennhold, K., Kim, H. J. & von Boehmer, H. Enhancement of antigen-specific Treg vaccination in vivo. Proc. Natl Acad. Sci. USA 107, 16246–16251 (2010).
Webster, K. E. et al. In vivo expansion of T reg cells with IL-2–mAb complexes: induction of resistance to EAE and long-term acceptance of islet allografts without immunosuppression. J. Exp. Med. 206, 751–760 (2009).
Koreth, J. et al. Interleukin-2 and regulatory T cells in graft-versus-host disease. N. Engl. J. Med. 365, 2055–2066 (2011).
Saadoun, D. et al. Regulatory T-cell responses to low-dose interleukin-2 in HCV-induced vasculitis. N. Engl. J. Med. 365, 2067–2077 (2011).
Miller, S. D., Turley, D. M. & Podojil, J. R. Antigen-specific tolerance strategies for the prevention and treatment of autoimmune disease. Nature Rev. Immunol. 7, 665–677 (2007).
Apostolou, I., Sarukhan, A., Klein, L. & von Boehmer, H. Origin of regulatory T cells with known specificity for antigen. Nature Immunol. 3, 756–763 (2002).
Jordan, M. S. et al. Thymic selection of CD4+CD25+ regulatory T cells induced by an agonist self-peptide. Nature Immunol. 2, 301–306 (2001).
Apostolou, I. & von Boehmer, H. In vivo instruction of suppressor commitment in naive T cells. J. Exp. Med. 199, 1401–1408 (2004).
Daniel, C., Ploegh, H. & von Boehmer, H. Antigen-specific induction of regulatory T cells in vivo and in vitro. Methods Mol. Biol. 707, 173–185 (2011).
Kretschmer, K. et al. Inducing and expanding regulatory T cell populations by foreign antigen. Nature Immunol. 6, 1219–1227 (2005).
Izcue, A., Coombes, J. L. & Powrie, F. Regulatory lymphocytes and intestinal inflammation. Annu. Rev. Immunol. 27, 313–338 (2009).
Gottschalk, R. A., Corse, E. & Allison, J. P. TCR ligand density and affinity determine peripheral induction of Foxp3 in vivo. J. Exp. Med. 207, 1701–1711 (2010).
Daniel, C., Weigmann, B., Bronson, R. & von Boehmer, H. Prevention of type 1 diabetes in mice by tolerogenic vaccination with a strong agonist insulin mimetope. J. Exp. Med. 208, 1501–1510 (2011).
Josefowicz, S. Z., Wilson, C. B. & Rudensky, A. Y. Cutting edge: TCR stimulation is sufficient for induction of Foxp3 expression in the absence of DNA methyltransferase 1. J. Immunol. 182, 6648–6652 (2009).
Klein, L., Khazaie, K. & von Boehmer, H. In vivo dynamics of antigen-specific regulatory T cells not predicted from behavior in vitro. Proc. Natl Acad. Sci. USA 100, 8886–8891 (2003).
Rubtsov, Y. P. et al. Stability of the regulatory T cell lineage in vivo. Science 329, 1667–1671 (2010).
van der Merwe, P. A. & Dushek, O. Mechanisms for T cell receptor triggering. Nature Rev. Immunol. 11, 47–55 (2011).
Merkenschlager, M. & von Boehmer, H. PI3 kinase signalling blocks Foxp3 expression by sequestering Foxo factors. J. Exp. Med. 207, 1347–1350 (2010).
Sauer, S. et al. T cell receptor signaling controls Foxp3 expression via PI3K, Akt, and mTOR. Proc. Natl Acad. Sci. USA 105, 7797–7802 (2008).
Haxhinasto, S., Mathis, D. & Benoist, C. The AKT–mTOR axis regulates de novo differentiation of CD4+Foxp3+ cells. J. Exp. Med. 205, 565–574 (2008).
Gavin, M. A. et al. Single-cell analysis of normal and FOXP3-mutant human T cells: FOXP3 expression without regulatory T cell development. Proc. Natl Acad. Sci. USA 103, 6659–6664 (2006).
Allan, S. E., Song-Zhao, G. X., Abraham, T., McMurchy, A. N. & Levings, M. K. Inducible reprogramming of human T cells into Treg cells by a conditionally active form of FOXP3. Eur. J. Immunol. 38, 3282–3289 (2008).
Hoffmann, P. et al. Loss of FOXP3 expression in natural human CD4+CD25+ regulatory T cells upon repetitive in vitro stimulation. Eur. J. Immunol. 39, 1088–1097 (2009).
Sullivan, S. P. et al. Dissolving polymer microneedle patches for influenza vaccination. Nature Med. 16, 915–920 (2010).
Gupta, J., Felner, E. I. & Prausnitz, M. R. Rapid pharmacokinetics of intradermal insulin administered using microneedles in type 1 diabetes subjects. Diabetes Technol. Ther. 13, 451–456 (2011).
Wohlfert, E. A., Gorelik, L., Mittler, R., Flavell, R. A. & Clark, R. B. Cutting edge: deficiency in the E3 ubiquitin ligase Cbl-b results in a multifunctional defect in T cell TGF-β sensitivity in vitro and in vivo. J. Immunol. 176, 1316–1320 (2006).
Delgoffe, G. M. et al. The kinase mTOR regulates the differentiation of helper T cells through the selective activation of signaling by mTORC1 and mTORC2. Nature Immunol. 12, 295–303 (2011).
Dennis, P. B., Fumagalli, S. & Thomas, G. Target of rapamycin (TOR): balancing the opposing forces of protein synthesis and degradation. Curr. Opin. Genet. Dev. 9, 49–54 (1999).
Schmelzle, T. & Hall, M. N. TOR, a central controller of cell growth. Cell 103, 253–262 (2000).
Cobbold, S. P. et al. Infectious tolerance via the consumption of essential amino acids and mTOR signaling. Proc. Natl Acad. Sci. USA 106, 12055–12060 (2009).
Battaglia, M., Stabilini, A. & Roncarolo, M. G. Rapamycin selectively expands CD4+CD25+FoxP3+ regulatory T cells. Blood 105, 4743–4748 (2005).
Mancini, M., Petta, S., Martinelli, G., Barbieri, E. & Santucci, M. A. RAD 001 (everolimus) prevents mTOR and Akt late re-activation in response to imatinib in chronic myeloid leukemia. J. Cell Biochem. 109, 320–328 (2010).
Powell, J. D. & Delgoffe, G. M. The mammalian target of rapamycin: linking T cell differentiation, function, and metabolism. Immunity 33, 301–311 (2010).
Sarbassov, D. D. et al. Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Mol. Cell 22, 159–168 (2006).
Zeng, Z. et al. Rapamycin derivatives reduce mTORC2 signaling and inhibit AKT activation in AML. Blood 109, 3509–3512 (2007).
Harada, Y. et al. Transcription factors Foxo3a and Foxo1 couple the E3 ligase Cbl-b to the induction of Foxp3 expression in induced regulatory T cells. J. Exp. Med. 207, 1381–1391 (2010).
Ouyang, W. et al. Foxo proteins cooperatively control the differentiation of Foxp3+ regulatory T cells. Nature Immunol. 11, 618–627 (2010).
Benjamin, D., Colombi, M., Moroni, C. & Hall, M. N. Rapamycin passes the torch: a new generation of mTOR inhibitors. Nature Rev. Drug Discov. 10, 868–880 (2011).
Hedrick, S. M. The cunning little vixen: Foxo and the cycle of life and death. Nature Immunol. 10, 1057–1063 (2009).
Link, W. et al. Chemical interrogation of FOXO3a nuclear translocation identifies potent and selective inhibitors of phosphoinositide 3-kinases. J. Biol. Chem. 284, 28392–28400 (2009).
Josefowicz, S. Z., Lu, L. F. & Rudensky, A. Y. Regulatory T cells: mechanisms of differentiation and function. Annu. Rev. Immunol. 30, 531–564 (2012).
Wang, Y. et al. Regulatory T cells require mammalian target of rapamycin signaling to maintain both homeostasis and alloantigen-driven proliferation in lymphocyte-replete mice. J. Immunol. 186, 2809–2818 (2011).
Matloubian, M. et al. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature 427, 355–360 (2004). This paper shows that mice with haematopoietic cells lacking S1PR1 do not harbour T cells in the periphery as mature T cells are unable to exit the thymus.
Liu, G., Yang, K., Burns, S., Shrestha, S. & Chi, H. The S1P1–mTOR axis directs the reciprocal differentiation of TH1 and Treg cells. Nature Immunol. 11, 1047–1056 (2010).
Liu, G. et al. The receptor S1P1 overrides regulatory T cell-mediated immune suppression through Akt–mTOR. Nature Immunol. 10, 769–777 (2009). References 92 and 93 show that FTY720 could also promote the generation of T Reg cells in vivo.
Zemann, B. et al. Sphingosine kinase type 2 is essential for lymphopenia induced by the immunomodulatory drug FTY720. Blood 107, 1454–1458 (2006).
Brinkmann, V. et al. The immune modulator FTY720 targets sphingosine 1-phosphate receptors. J. Biol. Chem. 277, 21453–21457 (2002).
Albert, R. et al. Novel immunomodulator FTY720 is phosphorylated in rats and humans to form a single stereoisomer. Identification, chemical proof, and biological characterization of the biologically active species and its enantiomer. J. Med. Chem. 48, 5373–5377 (2005).
Mandala, S. et al. Alteration of lymphocyte trafficking by sphingosine-1-phosphate receptor agonists. Science 296, 346–349 (2002).
Daniel, C. et al. FTY720 ameliorates Th1-mediated colitis in mice by directly affecting the functional activity of CD4+CD25+ regulatory T cells. J. Immunol. 178, 2458–2468 (2007).
Sawicka, E. et al. The sphingosine 1-phosphate receptor agonist FTY720 differentially affects the sequestration of CD4+/CD25+ T-regulatory cells and enhances their functional activity. J. Immunol. 175, 7973–7980 (2005).
Jo, E. et al. S1P1-selective in vivo-active agonists from high-throughput screening: off-the-shelf chemical probes of receptor interactions, signaling, and fate. Chem. Biol. 12, 703–715 (2005).
Wei, S. H. et al. Sphingosine 1-phosphate type 1 receptor agonism inhibits transendothelial migration of medullary T cells to lymphatic sinuses. Nature Immunol. 6, 1228–1235 (2005).
Shimizu, H. et al. KRP-203, a novel synthetic immunosuppressant, prolongs graft survival and attenuates chronic rejection in rat skin and heart allografts. Circulation 111, 222–229 (2005).
Brinkmann, V. et al. Fingolimod (FTY720): discovery and development of an oral drug to treat multiple sclerosis. Nature Rev. Drug Discov. 9, 883–897 (2010).
Davis, M. D., Clemens, J. J., Macdonald, T. L. & Lynch, K. R. Sphingosine 1-phosphate analogs as receptor antagonists. J. Biol. Chem. 280, 9833–9841 (2005).
Ohmori, T. et al. Sphingosine 1-phosphate induces contraction of coronary artery smooth muscle cells via S1P2. Cardiovasc. Res. 58, 170–177 (2003).
Sanna, M. G. et al. Enhancement of capillary leakage and restoration of lymphocyte egress by a chiral S1P1 antagonist in vivo. Nature Chem. Biol. 2, 434–441 (2006).
Marsolais, D. & Rosen, H. Chemical modulators of sphingosine-1-phosphate receptors as barrier-oriented therapeutic molecules. Nature Rev. Drug Discov. 8, 297–307 (2009).
Takabe, K., Paugh, S. W., Milstien, S. & Spiegel, S. “Inside-out” signaling of sphingosine-1-phosphate: therapeutic targets. Pharmacol. Rev. 60, 181–195 (2008).
Xiao, C. & Rajewsky, K. MicroRNA control in the immune system: basic principles. Cell 136, 26–36 (2009).
Cobb, B. S. et al. A role for Dicer in immune regulation. J. Exp. Med. 203, 2519–2527 (2006).
Cobb, B. S. et al. T cell lineage choice and differentiation in the absence of the RNase III enzyme Dicer. J. Exp. Med. 201, 1367–1373 (2005).
Chong, M. M., Rasmussen, J. P., Rudensky, A. Y. & Littman, D. R. The RNAseIII enzyme Drosha is critical in T cells for preventing lethal inflammatory disease. J. Exp. Med. 205, 2005–2017 (2008).
Liston, A., Lu, L. F., O'Carroll, D., Tarakhovsky, A. & Rudensky, A. Y. Dicer-dependent microRNA pathway safeguards regulatory T cell function. J. Exp. Med. 205, 1993–2004 (2008).
Lu, L. F. et al. Foxp3-dependent microRNA155 confers competitive fitness to regulatory T cells by targeting SOCS1 protein. Immunity 30, 80–91 (2009).
Burchill, M. A. et al. Linked T cell receptor and cytokine signaling govern the development of the regulatory T cell repertoire. Immunity 28, 112–121 (2008).
Burchill, M. A., Yang, J., Vogtenhuber, C., Blazar, B. R. & Farrar, M. A. IL-2 receptor β-dependent STAT5 activation is required for the development of Foxp3+ regulatory T cells. J. Immunol. 178, 280–290 (2007).
Yao, Z. et al. Nonredundant roles for Stat5a/b in directly regulating Foxp3. Blood 109, 4368–4375 (2007).
Lu, L. F. et al. Function of miR-146a in controlling Treg cell-mediated regulation of Th1 responses. Cell 142, 914–929 (2010). This paper highlights the crucial role of miR-146a in the maintenance of the suppressive function of T Reg cells in mice in vivo.
Hou, J. et al. MicroRNA-146a feedback inhibits RIG-I-dependent type I IFN production in macrophages by targeting TRAF6, IRAK1, and IRAK2. J. Immunol. 183, 2150–2158 (2009).
Taganov, K. D., Boldin, M. P., Chang, K. J. & Baltimore, D. NF-κB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proc. Natl Acad. Sci. USA 103, 12481–12486 (2006).
Ebert, P. J., Jiang, S., Xie, J., Li, Q. J. & Davis, M. M. An endogenous positively selecting peptide enhances mature T cell responses and becomes an autoantigen in the absence of microRNA miR-181a. Nature Immunol. 10, 1162–1169 (2009).
Li, Q. J. et al. miR-181a is an intrinsic modulator of T cell sensitivity and selection. Cell 129, 147–161 (2007).
Josefowicz, S. Z. & Rudensky, A. Control of regulatory T cell lineage commitment and maintenance. Immunity 30, 616–625 (2009).
Pardoll, D. M. The blockade of immune checkpoints in cancer immunotherapy. Nature Rev. Cancer 12, 252–264 (2012).
Mempel, T. R. et al. Regulatory T cells reversibly suppress cytotoxic T cell function independent of effector differentiation. Immunity 25, 129–141 (2006).
Sakaguchi, S., Sakaguchi, N., Asano, M., Itoh, M. & Toda, M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor α-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J. Immunol. 155, 1151–1164 (1995).
Pasare, C. & Medzhitov, R. Toll pathway-dependent blockade of CD4+CD25+ T cell-mediated suppression by dendritic cells. Science 299, 1033–1036 (2003).
Peng, G. et al. Toll-like receptor 8-mediated reversal of CD4+ regulatory T cell function. Science 309, 1380–1384 (2005).
Yang, Y., Huang, C. T., Huang, X. & Pardoll, D. M. Persistent Toll-like receptor signals are required for reversal of regulatory T cell-mediated CD8 tolerance. Nature Immunol. 5, 508–515 (2004).
Ghiringhelli, F. et al. Metronomic cyclophosphamide regimen selectively depletes CD4+CD25+ regulatory T cells and restores T and NK effector functions in end stage cancer patients. Cancer Immunol. Immunother. 56, 641–648 (2007).
Liu, W. M., Fowler, D. W., Smith, P. & Dalgleish, A. G. Pre-treatment with chemotherapy can enhance the antigenicity and immunogenicity of tumours by promoting adaptive immune responses. Br. J. Cancer 102, 115–123 (2010). References 130 and 131 support the concept that chemotherapy can promote the inhibition of T Reg cells, which could help induce anticancer immunity.
Zhu, Y., Liu, N., Xiong, S. D., Zheng, Y. J. & Chu, Y. W. CD4+Foxp3+ regulatory T-cell impairment by paclitaxel is independent of Toll-like receptor 4. Scand. J. Immunol. 73, 301–308 (2011).
Larmonier, N. et al. Imatinib mesylate inhibits CD4+ CD25+ regulatory T cell activity and enhances active immunotherapy against BCR-ABL− tumors. J. Immunol. 181, 6955–6963 (2008).
Adotevi, O. et al. A decrease of regulatory T cells correlates with overall survival after sunitinib-based antiangiogenic therapy in metastatic renal cancer patients. J. Immunother. 33, 991–998 (2010).
Xin, H. et al. Sunitinib inhibition of Stat3 induces renal cell carcinoma tumor cell apoptosis and reduces immunosuppressive cells. Cancer Res. 69, 2506–2513 (2009).
Pallandre, J. R. et al. Role of STAT3 in CD4+CD25+FOXP3+ regulatory lymphocyte generation: implications in graft-versus-host disease and antitumor immunity. J. Immunol. 179, 7593–7604 (2007).
Lee, H., Pal, S. K., Reckamp, K., Figlin, R. A. & Yu, H. STAT3: a target to enhance antitumor immune response. Curr. Top. Microbiol. Immunol. 344, 41–59 (2011).
Vanneman, M. & Dranoff, G. Combining immunotherapy and targeted therapies in cancer treatment. Nature Rev. Cancer 12, 237–251 (2012).
Korman, A. J., Peggs, K. S. & Allison, J. P. Checkpoint blockade in cancer immunotherapy. Adv. Immunol. 90, 297–339 (2006).
Peggs, K. S., Quezada, S. A., Chambers, C. A., Korman, A. J. & Allison, J. P. Blockade of CTLA-4 on both effector and regulatory T cell compartments contributes to the antitumor activity of anti-CTLA-4 antibodies. J. Exp. Med. 206, 1717–1725 (2009). This paper demonstrates that blockade of CTLA4 on effector as well as T Reg cells could permit both the enhancement of effector cell function as well as inhibition of T Reg cell-mediated immune suppression.
Leach, D. R., Krummel, M. F. & Allison, J. P. Enhancement of antitumor immunity by CTLA-4 blockade. Science 271, 1734–1736 (1996).
Dannull, J. et al. Enhancement of vaccine-mediated antitumor immunity in cancer patients after depletion of regulatory T cells. J. Clin. Invest. 115, 3623–3633 (2005).
de Vries, I. J. et al. Frequency of circulating Tregs with demethylated FOXP3 intron 1 in melanoma patients receiving tumor vaccines and potentially Treg-depleting agents. Clin. Cancer Res. 17, 841–848 (2011).
Jacobs, J. F. et al. Dendritic cell vaccination in combination with anti-CD25 monoclonal antibody treatment: a phase I/II study in metastatic melanoma patients. Clin. Cancer Res. 16, 5067–5078 (2010).
Litzinger, M. T. et al. IL-2 immunotoxin denileukin diftitox reduces regulatory T cells and enhances vaccine-mediated T-cell immunity. Blood 110, 3192–3201 (2007).
Rech, A. J. & Vonderheide, R. H. Clinical use of anti-CD25 antibody daclizumab to enhance immune responses to tumor antigen vaccination by targeting regulatory T cells. Ann. NY Acad. Sci. 1174, 99–106 (2009).
Curiel, T. J. et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nature Med. 10, 942–949 (2004).
Zhao, E. et al. Regulatory T cells in the bone marrow microenvironment in patients with prostate cancer. Oncoimmunology 1, 152–161 (2012).
Zarek, P. E. et al. A2A receptor signaling promotes peripheral tolerance by inducing T-cell anergy and the generation of adaptive regulatory T cells. Blood 111, 251–259 (2008).
Waickman, A. T. et al. Enhancement of tumor immunotherapy by deletion of the A2A adenosine receptor. Cancer Immunol. Immunother. 61, 917–926 (2012).
Diabetes Prevention Trial — Type 1 Diabetes Study Group. Effects of insulin in relatives of patients with type 1 diabetes mellitus. N. Engl. J. Med. 346, 1685–1691 (2002).
Harrison, L. C. et al. Pancreatic β-cell function and immune responses to insulin after administration of intranasal insulin to humans at risk for type 1 diabetes. Diabetes Care 27, 2348–2355 (2004).
Skyler, J. S. et al. Effects of oral insulin in relatives of patients with type 1 diabetes: The Diabetes Prevention Trial — Type 1. Diabetes Care 28, 1068–1076 (2005).
Nanto-Salonen, K. et al. Nasal insulin to prevent type 1 diabetes in children with HLA genotypes and autoantibodies conferring increased risk of disease: a double-blind, randomised controlled trial. Lancet 372, 1746–1755 (2008).
Burks, A. W. et al. Oral immunotherapy for treatment of egg allergy in children. N. Engl. J. Med. 367, 233–243 (2012).
Bonifacio, E., Achenbach, P., Pan, L. & Ziegler, A. G. Mucosal insulin vaccination for type 1 diabetes prevention. Exp. Clin. Endocrinol. Diabetes 116 (Suppl. 1), 26–29 (2008).
Luo, X., Herold, K. C. & Miller, S. D. Immunotherapy of type 1 diabetes: where are we and where should we be going? Immunity 32, 488–499 (2010).
Baecher-Allan, C., Brown, J. A., Freeman, G. J. & Hafler, D. A. CD4+CD25high regulatory cells in human peripheral blood. J. Immunol. 167, 1245–1253 (2001).
Dieckmann, D., Plottner, H., Berchtold, S., Berger, T. & Schuler, G. Ex vivo isolation and characterization of CD4+CD25+ T cells with regulatory properties from human blood. J. Exp. Med. 193, 1303–1310 (2001).
Jonuleit, H. et al. Identification and functional characterization of human CD4+CD25+ T cells with regulatory properties isolated from peripheral blood. J. Exp. Med. 193, 1285–1294 (2001).
Levings, M. K., Sangregorio, R. & Roncarolo, M. G. Human CD25+CD4+ T regulatory cells suppress naive and memory T cell proliferation and can be expanded in vitro without loss of function. J. Exp. Med. 193, 1295–1302 (2001).
Ng, W. F. et al. Human CD4+CD25+ cells: a naturally occurring population of regulatory T cells. Blood 98, 2736–2744 (2001).
Hori, S., Takahashi, T. & Sakaguchi, S. Control of autoimmunity by naturally arising regulatory CD4+ T cells. Adv. Immunol. 81, 331–371 (2003).
Roncador, G. et al. Analysis of FOXP3 protein expression in human CD4+CD25+ regulatory T cells at the single-cell level. Eur. J. Immunol. 35, 1681–1691 (2005).
Du, J., Huang, C., Zhou, B. & Ziegler, S. F. Isoform-specific inhibition of RORα-mediated transcriptional activation by human FOXP3. J. Immunol. 180, 4785–4792 (2008).
Bettelli, E., Dastrange, M. & Oukka, M. Foxp3 interacts with nuclear factor of activated T cells and NF-κB to repress cytokine gene expression and effector functions of T helper cells. Proc. Natl Acad. Sci. USA 102, 5138–5143 (2005).
Lopes, J. E. et al. Analysis of FOXP3 reveals multiple domains required for its function as a transcriptional repressor. J. Immunol. 177, 3133–3142 (2006).
Aarts-Riemens, T., Emmelot, M. E., Verdonck, L. F. & Mutis, T. Forced overexpression of either of the two common human Foxp3 isoforms can induce regulatory T cells from CD4+CD25− cells. Eur. J. Immunol. 38, 1381–1390 (2008).
Allan, S. E. et al. The role of 2 FOXP3 isoforms in the generation of human CD4+ Tregs. J. Clin. Invest. 115, 3276–3284 (2005).
Yagi, H. et al. Crucial role of FOXP3 in the development and function of human CD25+CD4+ regulatory T cells. Int. Immunol. 16, 1643–1656 (2004).
Cvetanovich, G. L. & Hafler, D. A. Human regulatory T cells in autoimmune diseases. Curr. Opin. Immunol. 22, 753–760 (2010).
Miyara, M. et al. Functional delineation and differentiation dynamics of human CD4+ T cells expressing the FoxP3 transcription factor. Immunity 30, 899–911 (2009).
Sakaguchi, S., Miyara, M., Costantino, C. M. & Hafler, D. A. FOXP3+ regulatory T cells in the human immune system. Nature Rev. Immunol. 10, 490–500 (2010).
Jaeckel, E., Lipes, M. A. & von Boehmer, H. Recessive tolerance to preproinsulin 2 reduces but does not abolish type 1 diabetes. Nature Immunol. 5, 1028–1035 (2004).
Nakayama, M. et al. Prime role for an insulin epitope in the development of type 1 diabetes in NOD mice. Nature 435, 220–223 (2005).
Nakayama, M. et al. Priming and effector dependence on insulin B:9-23 peptide in NOD islet autoimmunity. J. Clin. Invest. 117, 1835–1843 (2007).
Alleva, D. G. et al. A disease-associated cellular immune response in type 1 diabetics to an immunodominant epitope of insulin. J. Clin. Invest. 107, 173–180 (2001).
Daniel, D., Gill, R. G., Schloot, N. & Wegmann, D. Epitope specificity, cytokine production profile and diabetogenic activity of insulin-specific T cell clones isolated from NOD mice. Eur. J. Immunol. 25, 1056–1062 (1995).
Wegmann, D. R., Norbury-Glaser, M. & Daniel, D. Insulin-specific T cells are a predominant component of islet infiltrates in pre-diabetic NOD mice. Eur. J. Immunol. 24, 1853–1857 (1994).
Ziegler, A. G. & Nepom, G. T. Prediction and pathogenesis in type 1 diabetes. Immunity 32, 468–478 (2010).
Crawford, F. et al. Specificity and detection of insulin-reactive CD4+ T cells in type 1 diabetes in the nonobese diabetic (NOD) mouse. Proc. Natl Acad. Sci. USA 108, 16729–16734 (2011).
Corper, A. L. et al. A structural framework for deciphering the link between I-Ag7 and autoimmune diabetes. Science 288, 505–511 (2000).
Latek, R. R. et al. Structural basis of peptide binding and presentation by the type I diabetes-associated MHC class II molecule of NOD mice. Immunity 12, 699–710 (2000).
Manz, M. G. Human-hemato-lymphoid-system mice: opportunities and challenges. Immunity 26, 537–541 (2007).
Lee, K. H., Wucherpfennig, K. W. & Wiley, D. C. Structure of a human insulin peptide-HLA-DQ8 complex and susceptibility to type 1 diabetes. Nature Immunol. 2, 501–507 (2001).
Marodon, G. et al. High diversity of the immune repertoire in humanized NOD.SCID.γ c−/− mice. Eur. J. Immunol. 39, 2136–2145 (2009).
Shultz, L. D., Ishikawa, F. & Greiner, D. L. Humanized mice in translational biomedical research. Nature Rev. Immunol. 7, 118–130 (2007).
Acknowledgements
The studies were supported by the US National Institutes of Health Grant NIH-AI-53102 (to H.V.B.). C.D. was supported by a Leopoldina research fellowship (BMBF-LPD 9901/8-184) and is now supported by a Junior Research Group at Helmholtz Zentrum München, Germany.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Glossary
- Recessive tolerance
-
A form of passive tolerance involving clonal deletion (negative selection) and clonal inactivation (anergy), both of which represent cell-intrinsic mechanisms.
- Dominant tolerance
-
The active suppression of an immune response by suppressor cells including regulatory T (TReg) cells. By contrast, deletional tolerance and induction of anergy are forms of passive tolerance. Dominant tolerance is transferable to naive recipients, whereas passive tolerance is not.
- Negative selection
-
Also known as clonal deletion. The intrathymic elimination of CD4+CD8+ double-positive and single-positive thymocytes that express T cell receptors with a high affinity for self-antigens.
- Fork-head box P3
-
(FOXP3). Forkhead or winged helix transcription factor that is expressed primarily in regulatory T (TReg) cells and controls their function. Mutations in the Foxp3 gene result in life-threatening autoimmunity in humans as well as in experimental mice.
- Bystander suppression
-
A situation whereby regulatory T cells of a particular specificity are able to suppress several effector cells with different specificities when colocalized at the same antigen-presenting cell.
- Type 1 diabetes
-
A disorder caused by the autoimmune-mediated destruction of pancreatic islet β-cells, resulting in hyperglycaemia and insulin deficiency at diagnosis.
- TNF receptor-associated factor 6
-
(TRAF6). A protein that is associated with, and mediates, signal transduction from members of the tumour necrosis factor (TNF) receptor superfamily, and also from members of the Toll-like receptor and interleukin-1receptor family. TRAF6 also interacts with various protein kinases, including interleukin-1 receptor-associated kinase 1 (IRAK1).
- IL-1R-associated kinase 1
-
(IRAK1). Along with IRAK2, IRAK1 is a putative serine/threonine kinase that becomes associated with the interleukin-1 receptor (IL-1R) upon stimulation and is responsible for IL-1-induced upregulation of the transcription factor nuclear factor-κB.
- Cytotoxic T lymphocyte antigen 4
-
(CTLA4). A member of the immunoglobulin superfamily. CTLA4 is expressed on the surface of T helper cells and transmits an inhibitory signal to T cells.
- CC motif chemokine 22
-
(CCL22). A protein that is encoded by the CCL22 gene in humans. CCL22 is secreted by dendritic cells and macrophages, and elicits its effects on its target cells by interacting with cell surface chemokine receptors such as CC chemokine receptor 4.
- CXC chemokine receptor 4
-
(CXCR4). An α-chemokine receptor that is specific for CXC motif chemokine ligand 12 (CXCL12; also known as SDF1), a molecule with potent chemotactic activity for lymphocytes.
- CXC motif chemokine ligand 12
-
(CXCL12; also known as SDF1). A small cytokine belonging to the chemokine family, members of which are often induced by pro-inflammatory stimuli such as lipopolysaccharides, tumour necrosis factor or interleukin-1.
Rights and permissions
About this article
Cite this article
von Boehmer, H., Daniel, C. Therapeutic opportunities for manipulating TReg cells in autoimmunity and cancer. Nat Rev Drug Discov 12, 51–63 (2013). https://doi.org/10.1038/nrd3683
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrd3683
This article is cited by
-
miRNA Regulation of T Cells in Islet Autoimmunity and Type 1 Diabetes
Current Diabetes Reports (2020)
-
Critical role of OX40 signaling in the TCR-independent phase of human and murine thymic Treg generation
Cellular & Molecular Immunology (2019)
-
PI3Kδ inhibition modulates regulatory and effector T-cell differentiation and function in chronic lymphocytic leukemia
Leukemia (2019)
-
miRNA142-3p targets Tet2 and impairs Treg differentiation and stability in models of type 1 diabetes
Nature Communications (2019)
-
Mevalonate promotes differentiation of regulatory T cells
Journal of Molecular Medicine (2019)