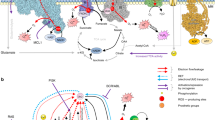
Key Points
-
Mitochondria contribute to the generation of ATP through oxidative phosphorylation, but they also participate in biosynthetic, metabolic and signalling functions in the cell. Some of the signalling functions are mediated by reactive oxygen species (ROS) that are generated by the electron transport chain. Alterations in mitochondrial ROS generation have been linked to a wide range of tumour cell types.
-
Mitochondria generate ROS when electrons residing on flavin groups, iron–sulphur centres or other electron transport 'way-stations' are diverted to O2, generating superoxide. Diverse 'antioxidant enzymes' scavenge ROS and/or reverse the effects of ROS on proteins, lipids and DNA, thereby limiting the scope of oxidative damage or redox signalling.
-
Mitochondrial ROS generation can be important in cancer because it activates cellular redox signalling that drives proliferative responses and triggers activation of transcription factors that promote tumorigenesis and survival, such as hypoxia-inducible factors (HIFs). Hypoxia triggers a paradoxical increase in the release of ROS from complex III to the mitochondrial intermembrane space, facilitating signalling, cell survival and proliferation.
-
Mitochondrial DNA can be damaged by ROS, and mutant mitochondrial proteins can augment ROS generation, creating a vicious cycle that contributes to cancer initiation or progression. Mitochondrial DNA mutations have been linked to a wide range of cancer types. In some cases, mitochondrial DNA mutations regulate the tumorigenic phenotype through their effect on ROS generation.
-
Mitochondrial ROS can contribute to genomic instability, and can contribute to the activation of mitochondria-dependent cell death pathways. However, a fuller understanding of the how altered mitochondrial ROS generation contributes to cancer progression is needed.
-
Oncogenes such as KRAS and MYC drive tumorigenesis in part by augmenting mitochondrial ROS generation.
-
As many tumour cells benefit from mitochondria-derived redox signalling, a useful therapeutic approach could revolve around the inhibition of tumour-promoting mitochondrial ROS signalling without interfering with ATP production. Such an approach could limit the ability of cells to activate protective responses, leaving them vulnerable to cytotoxic agents.
Abstract
Mitochondria cooperate with their host cells by contributing to bioenergetics, metabolism, biosynthesis, and cell death or survival functions. Reactive oxygen species (ROS) generated by mitochondria participate in stress signalling in normal cells but also contribute to the initiation of nuclear or mitochondrial DNA mutations that promote neoplastic transformation. In cancer cells, mitochondrial ROS amplify the tumorigenic phenotype and accelerate the accumulation of additional mutations that lead to metastatic behaviour. As mitochondria carry out important functions in normal cells, disabling their function is not a feasible therapy for cancer. However, ROS signalling contributes to proliferation and survival in many cancers, so the targeted disruption of mitochondria-to-cell redox communication represents a promising avenue for future therapy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Embley, T. M. & Martin, W. Eukaryotic evolution, changes and challenges. Nature 440, 623–630 (2006).
Martin, W., Hoffmeister, M., Rotte, C. & Henze, K. An overview of endosymbiotic models for the origins of eukaryotes, their ATP-producing organelles (mitochondria and hydrogenosomes), and their heterotrophic lifestyle. Biol. Chem. 382, 1521–1539 (2001).
Timmis, J. N., Ayliffe, M. A., Huang, C. Y. & Martin, W. Endosymbiotic gene transfer: organelle genomes forge eukaryotic chromosomes. Nature Rev. Genet. 5, 123–135 (2004).
Adams, K. L. & Palmer, J. D. Evolution of mitochondrial gene content: gene loss and transfer to the nucleus. Mol. Phylogenet. Evol. 29, 380–395 (2003).
Murphy, M. P. et al. Unraveling the biological roles of reactive oxygen species. Cell. Metab. 13, 361–366 (2011).
Chance, B., Sies, H. & Boveris, A. Hydroperoxide metabolism in mammalian organs. Physiol. Rev. 59, 527–605 (1979).
Quinlan, C. L. et al. The 2-oxoacid dehydrogenase complexes in mitochondria can produce superoxide/hydrogen peroxide at much higher rates than complex I. J. Biol. Chem. 289, 8312–8325 (2014).
Gardner, P. R. Superoxide-driven aconitase FE-S center cycling. Biosci. Rep. 17, 33–42 (1997).
Guzy, R. D. & Schumacker, P. T. Oxygen sensing by mitochondria at complex III: the paradox of increased reactive oxygen species during hypoxia. Exp. Physiol. 91, 807–819 (2006).
Fabian, M. & Palmer, G. Hydrogen peroxide is not released following reaction of cyanide with several catalytically important derivatives of cytochrome c oxidase. FEBS Lett. 422, 1–4 (1998).
Han, D., Antunes, F., Canali, R., Rettori, D. & Cadenas, E. Voltage-dependent anion channels control the release of the superoxide anion from mitochondria to cytosol. J. Biol. Chem. 278, 5557–5563 (2003).
Bienert, G. P. et al. Specific aquaporins facilitate the diffusion of hydrogen peroxide across membranes. J. Biol. Chem. 282, 1183–1192 (2007).
Waypa, G. B. et al. Hypoxia triggers subcellular compartmental redox signaling in vascular smooth muscle cells. Circ. Res. 106, 526–535 (2010). This study demonstrates how hypoxia-induced changes in mitochondrial ROS generation affect redox status in subcellular compartments differently.
Sabharwal, S. S., Waypa, G. B., Marks, J. D. & Schumacker, P. T. Peroxiredoxin-5 targeted to the mitochondrial intermembrane space attenuates hypoxia-induced reactive oxygen species signalling. Biochem. J. 456, 337–346 (2013).
Mills, G. C. Hemoglobin catabolism. I. Glutathione peroxidase, an erythrocyte enzyme which protects hemoglobin from oxidative breakdown. J. Biol. Chem. 229, 189–197 (1957).
Morgan, B. et al. Multiple glutathione disulfide removal pathways mediate cytosolic redox homeostasis. Nature Chem. Biol. 9, 119–125 (2013).
Chae, H. Z. & Rhee, S. G. A thiol-specific antioxidant and sequence homology to various proteins of unknown function. Biofactors 4, 177–180 (1994).
Kim, K., Kim, I. H., Lee, K. Y., Rhee, S. G. & Stadtman, E. R. The isolation and purification of a specific “protector” protein which inhibits enzyme inactivation by a thiol/Fe(III)/O2 mixed-function oxidation system. J. Biol. Chem. 263, 4704–4711 (1988).
Rhee, S. G., Chae, H. Z. & Kim, K. Peroxiredoxins: A historical overview and speculative preview of novel mechanisms and emerging concepts in cell signaling. Free Radic. Biol. Med. 38, 1543–1552 (2005).
Fan, J. et al. Quantitative flux analysis reveals folate-dependent NADPH production. Nature 510, 298–302 (2014).
Lewis, C. A. et al. Tracing compartmentalized NADPH metabolism in the cytosol and mitochondria of mammalian cells. Mol. Cell 55, 253–263 (2014).
Holmgren, A. Thioredoxin and glutaredoxin systems. J. Biol. Chem. 264, 13963–13966 (1989).
Rhee, S. G., Jeong, W., Chang, T. S. & Woo, H. A. Sulfiredoxin, the cysteine sulfinic acid reductase specific to 2-Cys peroxiredoxin: its discovery, mechanism of action, and biological significance. Kidney Int. Suppl. S3–S8 (2007).
Arner, E. S. & Holmgren, A. The thioredoxin system in cancer. Semin. Cancer Biol. 16, 420–426 (2006). This excellent review summarizes the role of thioredoxin and its contributions to the cellular phenotype of cancer cells.
Padmanabhan, B. et al. Structural basis for defects of Keap1 activity provoked by its point mutations in lung cancer. Mol. Cell 21, 689–700 (2006).
Singh, A., Bodas, M., Wakabayashi, N., Bunz, F. & Biswal, S. Gain of Nrf2 function in non-small-cell lung cancer cells confers radioresistance. Antioxid. Redox. Signal. 13, 1627–1637 (2010).
Shibata, T. et al. Cancer related mutations in NRF2 impair its recognition by Keap1-Cul3 E3 ligase and promote malignancy. Proc. Natl Acad. Sci. USA 105, 13568–13573 (2008).
Sullivan, L. B. et al. The proto-oncometabolite fumarate binds glutathione to amplify ROS-dependent signaling. Mol. Cell 51, 236–248 (2013). This study examines the mechanisms by which FH deficiency drives tumour cell behaviour in a redox-dependent manner.
Vander Heiden, M. G., Cantley, L. C. & Thompson, C. B. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science 324, 1029–1033 (2009). This excellent review summarizes the role of the Warburg effect on cell proliferation.
Le, A. et al. Tumorigenicity of hypoxic respiring cancer cells revealed by a hypoxia-cell cycle dual reporter. Proc. Natl Acad. Sci. USA 111, 12486–12491 (2014).
Waypa, G. B. et al. Superoxide generated at mitochondrial complex III triggers acute responses to hypoxia in the pulmonary circulation. Am. J. Respir. Crit. Care Med. 187, 424–432 (2013).
Farrow, K. N. et al. Brief hyperoxia increases mitochondrial oxidation and increases phosphodiesterase 5 activity in fetal pulmonary artery smooth muscle cells. Antioxid. Redox. Signal. 17, 460–470 (2012).
Chandel, N. S. et al. Mitochondrial reactive oxygen species trigger hypoxia-induced transcription. Proc. Natl Acad. Sci. USA 95, 11715–11720 (1998). This study was the first to demonstrate that mitochondria-derived ROS can regulate transcription through their control of HIF1α stability.
Waypa, G. B. et al. Increases in mitochondrial reactive oxygen species trigger hypoxia-induced calcium responses in pulmonary artery smooth muscle cells. Circ. Res. 99, 970–978 (2006).
Chi, A. Y., Waypa, G. B., Mungai, P. T. & Schumacker, P. T. Prolonged hypoxia increases ROS signaling and RhoA activation in pulmonary artery smooth muscle and endothelial cells. Antioxid. Redox. Signal. 12, 603–610 (2010).
Guzy, R. D. et al. Mitochondrial complex III is required for hypoxia-induced ROS production and cellular oxygen sensing. Cell. Metab. 1, 401–408 (2005). This study demonstrated the importance of mitochondrial complex III in hypoxia-induced ROS generation that controls HIF1α stability.
Mansfield, K. D. et al. Mitochondrial dysfunction resulting from loss of cytochrome c impairs cellular oxygen sensing and hypoxic HIF-α activation. Cell. Metab. 1, 393–399 (2005).
Bell, E. L., Emerling, B. M. & Chandel, N. S. Mitochondrial regulation of oxygen sensing. Mitochondrion. 5, 322–332 (2005).
Weinberg, F. et al. Mitochondrial metabolism and ROS generation are essential for Kras-mediated tumorigenicity. Proc. Natl Acad. Sci. USA 107, 8788–8793 (2010). This study examines the role of mitochondrial ROS signalling in the tumorigenic behaviour induced by KRAS activation.
Sanjuan-Pla, A. et al. A targeted antioxidant reveals the importance of mitochondrial reactive oxygen species in the hypoxic signaling of HIF-1α. FEBS Lett. 579, 2669–2674 (2005).
Hamanaka, R. B. et al. Mitochondrial reactive oxygen species promote epidermal differentiation and hair follicle development. Sci. Signal. 6, ra8 (2013).
Woo, D. K. et al. Mitochondrial genome instability and ROS enhance intestinal tumorigenesis in APCMin/+ mice. Am. J. Pathol. 180, 24–31 (2012).
Brandon, M., Baldi, P. & Wallace, D. C. Mitochondrial mutations in cancer. Oncogene 25, 4647–4662 (2006). This is an excellent review of the evidence linking mtDNA mutations and cancer.
Ishikawa, K. et al. ROS-generating mitochondrial DNA mutations can regulate tumor cell metastasis. Science 320, 661–664 (2008). This study demonstrates that mtDNA mutations can amplify tumour progression by increasing cellular ROS generation.
Alexeyev, M., Shokolenko, I., Wilson, G. & Ledoux, S. The maintenance of mitochondrial DNA integrity—critical analysis and update. Cold Spring Harb. Perspect. Biol. 5, a012641 (2013).
Larsen, N. B., Rasmussen, M. & Rasmussen, L. J. Nuclear and mitochondrial DNA repair: similar pathways? Mitochondrion 5, 89–108 (2005).
He, Y. et al. Heteroplasmic mitochondrial DNA mutations in normal and tumour cells. Nature 464, 610–614 (2010).
Clayton, D. A. & Vinograd, J. Circular dimer and catenate forms of mitochondrial DNA in human leukaemic leucocytes. Nature 216, 652–657 (1967).
Clayton, D. A. & Vinograd, J. Complex mitochondrial DNA in leukemic and normal human myeloid cells. Proc. Natl Acad. Sci. USA 62, 1077–1084 (1969).
Polyak, K. et al. Somatic mutations of the mitochondrial genome in human colorectal tumours. Nature Genet. 20, 291–293 (1998).
Kulawiec, M., Salk, J. J., Ericson, N. G., Wanagat, J. & Bielas, J. H. Generation, function, and prognostic utility of somatic mitochondrial DNA mutations in cancer. Environ. Mol. Mutagen 51, 427–439 (2010).
Chatterjee, A., Mambo, E. & Sidransky, D. Mitochondrial DNA mutations in human cancer. Oncogene 25, 4663–4674 (2006).
Coller, H. A. et al. High frequency of homoplasmic mitochondrial DNA mutations in human tumors can be explained without selection. Nature Genet. 28, 147–150 (2001).
Zhidkov, I., Livneh, E. A., Rubin, E. & Mishmar, D. MtDNA mutation pattern in tumors and human evolution are shaped by similar selective constraints. Genome Res. 19, 576–580 (2009).
Linnartz, B., Anglmayer, R. & Zanssen, S. Comprehensive scanning of somatic mitochondrial DNA alterations in acute leukemia developing from myelodysplastic syndromes. Cancer Res. 64, 1966–1971 (2004). This study tracked the association between the increase in mtDNA mutations over time and the progression from myelodysplastic syndrome to acute myeloid leukaemia in patients.
Kirches, E. et al. High frequency of mitochondrial DNA mutations in glioblastoma multiforme identified by direct sequence comparison to blood samples. Int. J. Cancer 93, 534–538 (2001).
Petros, J. A. et al. mtDNA mutations increase tumorigenicity in prostate cancer. Proc. Natl Acad. Sci. USA 102, 719–724 (2005). This study used cellular cybrids to examine the role of mtDNA mutations on ROS generation and tumorigenicity in prostate cancer cells.
Trounce, I., Neill, S. & Wallace, D. C. Cytoplasmic transfer of the mtDNA nt 8993 T-->G (ATP6) point mutation associated with Leigh syndrome into mtDNA-less cells demonstrates cosegregation with a decrease in state III respiration and ADP/O ratio. Proc. Natl Acad. Sci. USA 91, 8334–8338 (1994).
Mattiazzi, M. et al. The mtDNA T8993G (NARP) mutation results in an impairment of oxidative phosphorylation that can be improved by antioxidants. Hum. Mol. Genet. 13, 869–879 (2004).
Shidara, Y. et al. Positive contribution of pathogenic mutations in the mitochondrial genome to the promotion of cancer by prevention from apoptosis. Cancer Res. 65, 1655–1663 (2005).
Vazquez, F. et al. PGC1α expression defines a subset of human melanoma tumors with increased mitochondrial capacity and resistance to oxidative stress. Cancer Cell 23, 287–301 (2013).
Namslauer, I. & Brzezinski, P. A mitochondrial DNA mutation linked to colon cancer results in proton leaks in cytochrome c oxidase. Proc. Natl Acad. Sci. USA 106, 3402–3407 (2009).
Samper, E., Nicholls, D. G. & Melov, S. Mitochondrial oxidative stress causes chromosomal instability of mouse embryonic fibroblasts. Aging Cell 2, 277–285 (2003).
VanRemmen, H. et al. Life-long reduction in MnSOD activity results in increased DNA damage and higher incidence of cancer but does not accelerate aging. Physiol. Genom. 16, 29–37 (2003).
Liu, L., Trimarchi, J. R., Smith, P. J. & Keefe, D. L. Mitochondrial dysfunction leads to telomere attrition and genomic instability. Aging Cell 1, 40–46 (2002).
Martinez-Outschoorn, U. E. et al. Oxidative stress in cancer associated fibroblasts drives tumor-stroma co-evolution: A new paradigm for understanding tumor metabolism, the field effect and genomic instability in cancer cells. Cell Cycle 9, 3256–3276 (2010).
Degan, P. et al. In vivo accumulation of 8-hydroxy-2′-deoxyguanosine in DNA correlates with release of reactive oxygen species in Fanconi's anaemia families. Carcinogenesis 16, 735–741 (1995).
Ponte, F. et al. Improvement of genetic stability in lymphocytes from Fanconi anemia patients through the combined effect of α-lipoic acid and N-acetylcysteine. Orphanet. J. Rare. Dis. 7, 28 (2012).
Radisky, D. C. et al. Rac1b and reactive oxygen species mediate MMP-3-induced EMT and genomic instability. Nature 436, 123–127 (2005).
Gerasimenko, J. V. et al. Menadione-induced apoptosis: roles of cytosolic Ca2+ elevations and the mitochondrial permeability transition pore. J. Cell Sci. 115, 485–497 (2002).
Bernardi, P. Mitochondrial transport of cations: Channels, exchangers, and permeability transition. Physiol. Rev. 79, 1127–1155 (1999).
Giorgio, V. et al. Dimers of mitochondrial ATP synthase form the permeability transition pore. Proc. Natl Acad. Sci. USA 110, 5887–5892 (2013).
Baines, C. P. et al. Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature 434, 658–662 (2005).
Loor, G. et al. Mitochondrial oxidant stress triggers cell death in simulated ischemia-reperfusion. Biochim. Biophys. Acta 1813, 1382–1394 (2011).
Schriewer, J. M., Peek, C. B., Bass, J. & Schumacker, P. T. ROS-mediated, PARP activity undermines mitochondrial function after permeability transition pore opening during myocardial ischemia-reperfusion. J. Am. Heart Assoc. 2, e000159 (2013).
Dewhirst, M. W., Cao, Y. & Moeller, B. Cycling hypoxia and free radicals regulate angiogenesis and radiotherapy response. Nature Rev. Cancer 8, 425–437 (2008).
Trachootham, D. et al. Selective killing of oncogenically transformed cells through a ROS-mediated mechanism by β-phenylethyl isothiocyanate. Cancer Cell 10, 241–252 (2006).
Lee, S. R. et al. Reversible inactivation of the tumor suppressor PTEN by H2O2. J. Biol. Chem. 277, 20336–20342 (2002). This study demonstrated how H 2 O 2 generation can lead to the reversible inactivation of the lipid phosphatase PTEN, with important implications for cancer cell growth driven by excessive ROS signalling.
Kwon, J. et al. Reversible oxidation and inactivation of the tumor suppressor PTEN in cells stimulated with peptide growth factors. Proc. Natl Acad. Sci. USA 101, 16419–16424 (2004).
Gao, P. et al. HIF-dependent antitumorigenic effect of antioxidants in vivo. Cancer Cell 12, 230–238 (2007).
Semenza, G. L. Defining the role of hypoxia-inducible factor 1 in cancer biology and therapeutics. Oncogene 29, 625–634 (2010). This is an excellent review of the role of HIF1 in cancer.
Keith, B. & Simon, M. C. Hypoxia-inducible factors, stem cells, and cancer. Cell 129, 465–472 (2007).
Semenza, G. L. Regulation of cancer cell metabolism by hypoxia-inducible factor 1. Semin. Cancer Biol. 19, 12–16 (2009).
Huang, L. E., Gu, J., Schau, M. & Bunn, H. F. Regulation of hypoxia-inducible factor 1α is mediated by an O2-dependent degradation domain via the ubiquitin-proteasome pathway. Proc. Natl Acad. Sci. USA 95, 7987–7992 (1998). This is a classic paper describing the regions of the HIF1α protein that confer sensitivity to hypoxia.
Epstein, A. C. et al. C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell 107, 43–54 (2001). This study demonstrates the importance of SDH mutations in the formation of hereditary paragangliomas, thereby linking mitochondria to tumorigenic behaviour.
Ivan, M. et al. HIFα targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science 292, 464–468 (2001).
Maxwell, P. H. et al. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature 399, 271–275 (1999).
Chandel, N. S. et al. Reactive oxygen species generated at mitochondrial Complex III stabilize HIF-1-α during hypoxia: A mechanism of O2 sensing. J. Biol. Chem. 275, 25130–25138 (2000).
Brunelle, J. K. et al. Oxygen sensing requires mitochondrial ROS but not oxidative phosphorylation. Cell. Metab. 1, 409–414 (2005).
Selak, M. A. et al. Succinate links TCA cycle dysfunction to oncogenesis by inhibiting HIF-α prolyl hydroxylase. Cancer Cell 7, 77–85 (2005).
Rustin, P. & Roetig, A. Inborn errors of complex II - Unusual human mitochondrial diseases. Biochim. Biophys. Acta 1553, 117–122 (2002).
Burnichon, N. et al. SDHA is a tumor suppressor gene causing paraganglioma. Hum. Mol. Genet. 19, 3011–3020 (2010).
Ackrell, B. A. C. Progress in understanding structure-function relationships in respiratory chain complex II. FEBS Lett. 466, 1–5 (2000).
Baysal, B. E. et al. Mutations in SDHD, a mitochondrial complex II gene, in hereditary paraganglioma. Science 287, 848–851 (2000).
Gimenez-Roqueplo, A. P. et al. The R22X mutation of the SDHD gene in hereditary paraganglioma abolishes the enzymatic activity of complex II in the mitochondrial respiratory chain and activates the hypoxia pathway. Am. J. Hum. Genet. 69, 1186–1197 (2001).
Dekker, P. B. D. et al. SDHD mutations in head and neck paragangliomas result in destabilization of complex II in the mitochondrial respiratory chain with loss of enzymatic activity and abnormal mitochondrial morphology. J. Pathol. 201, 480–486 (2003).
Astrom, K., Cohen, J. E., Willett-Brozick, J. E., Aston, C. E. & Baysal, B. E. Altitude is a phenotypic modifier in hereditary paraganglioma type 1: evidence for an oxygen-sensing defect. Hum. Genet. 113, 228–237 (2003).
Guzy, R. D., Sharma, B., Bell, E., Chandel, N. S. & Schumacker, P. T. Loss of SdhB, but not SdhA, subunit of Complex. II triggers ROS-dependent HIF activation and tumorigenesis. Mol. Cell. Biol. 28, 718–731 (2007).
Owens, K. M. et al. Genomic instability induced by mutant succinate dehydrogenase subunit D (SDHD) is mediated by O2-• and H2O2 . Free Radic. Biol. Med. 52, 160–166 (2012).
Tomlinson, I. P. et al. Germline mutations in FH predispose to dominantly inherited uterine fibroids, skin leiomyomata and papillary renal cell cancer. Nature Genet. 30, 406–410 (2002).
Pollard, P. J. et al. Accumulation of Krebs cycle intermediates and over-expression of HIF1α in tumours which result from germline FH and SDH mutations. Hum. Mol. Genet. 14, 2231–2239 (2005).
Isaacs, J. S. et al. HIF overexpression correlates with biallelic loss of fumarate hydratase in renal cancer: novel role of fumarate in regulation of HIF stability. Cancer Cell 8, 143–153 (2005).
Sudarshan, S. et al. Fumarate hydratase deficiency in renal cancer induces glycolytic addiction and hypoxia-inducible transcription factor 1α stabilization by glucose-dependent generation of reactive oxygen species. Mol. Cell. Biol. 29, 4080–4090 (2009).
Mullen, A. R. et al. Reductive carboxylation supports growth in tumour cells with defective mitochondria. Nature 481, 385–388 (2012).
Semenza, G. L. HIF-1 mediates metabolic responses to intratumoral hypoxia and oncogenic mutations. J. Clin. Invest. 123, 3664–3671 (2013).
Hu, Y. et al. K-rasG12V transformation leads to mitochondrial dysfunction and a metabolic switch from oxidative phosphorylation to glycolysis. Cell Res. 22, 399–412 (2012).
Dang, C. V. et al. Function of the c-Myc oncogenic transcription factor. Exp. Cell Res. 253, 63–77 (1999).
Wise, D. R. et al. Myc regulates a transcriptional program that stimulates mitochondrial glutaminolysis and leads to glutamine addiction. Proc. Natl Acad. Sci. USA 105, 18782–18787 (2008).
Li, F. et al. Myc stimulates nuclearly encoded mitochondrial genes and mitochondrial biogenesis. Mol. Cell. Biol. 25, 6225–6234 (2005).
Shim, H. et al. c-Myc transactivation of LDH-A: implications for tumor metabolism and growth. Proc. Natl Acad. Sci. USA 94, 6658–6663 (1997).
Chandel, N. S., Trzyna, W. C., McClintock, D. S. & Schumacker, P. T. Role of oxidants in NF-κB activation and TNF-α gene transcription induced by hypoxia and endotoxin. J. Immunol. 165, 1013–1021 (2000).
Schumacker, P. T. Reactive oxygen species in cancer cells: live by the sword, die by the sword. Cancer Cell 10, 175–176 (2006).
Hashemy, S. I., Ungerstedt, J. S., Avval, F. Z. & Holmgren, A. Motexafin gadolinium, a tumor-selective drug targeting thioredoxin reductase and ribonucleotide reductase. J. Biol. Chem. 281, 10691–10697 (2006).
Goodman, M., Bostick, R. M., Kucuk, O. & Jones, D. P. Clinical trials of antioxidants as cancer prevention agents: past, present, and future. Free Radic. Biol. Med. 51, 1068–1084 (2011).
Creagan, E. T. et al. Failure of high-dose vitamin C (ascorbic acid) therapy to benefit patients with advanced cancer. A controlled trial. N. Engl. J. Med. 301, 687–690 (1979).
Jung, H. J. et al. Terpestacin inhibits tumor angiogenesis by targeting UQCRB of mitochondrial complex III and suppressing hypoxia-induced reactive oxygen species production and cellular oxygen sensing. J. Biol. Chem. 285, 11584–11595 (2010).
Acknowledgements
The authors were supported by the US National Institutes of Health (NIH) Grants HL35440 and HL122062.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- Mitochondria
-
Organelles within eukaryotic cells that participate in energy production, biosynthetic processes, redox regulation, cell survival, signalling and cell death pathways.
- ATP
-
(Adenosine triphosphate). A high-energy molecule that is hydrolysed by enzymes to provide the exergonic free energy required to carry out endergonic reactions.
- Hypoxia
-
A condition in which the molecular oxygen concentration is decreased relative to normal physiological levels.
- Reactive oxygen species
-
(ROS). Reactive molecules generated by the reduction of O2 with a single electron (superoxide), two electrons (hydrogen peroxide) or three electrons (hydroxyl radical).
- ROS signalling
-
(Reactive oxygen species signalling). A cellular signal transduction mechanism involving oxidation–reduction reactions, usually resulting in a reversible alteration of protein structure and function that elicits a subsequent cellular response. ROS signalling frequently involves redox alterations of cysteine thiol (SH) groups in proteins.
- Free radical
-
A molecule or atom containing an unpaired valence electron that renders it chemically reactive. Free radicals can potentially oxidize or reduce other molecules.
- Tricarboxylic acid cycle
-
(TCA cycle). A system within mitochondria that participates in intermediary metabolism involved in energy production, inter-conversion of metabolites, and synthesis of small molecules needed for lipid or protein synthesis.
- NADPH
-
A cofactor that is used by enzymes mediating electron transfer steps in energy production, lipid and nucleic acid synthesis, and the maintenance of intracellular oxidation–reduction status.
- Superoxide dismutases
-
A family of enzymes that redistribute electrons between two superoxide anions to form a single molecule of hydrogen peroxide.
- Hypoxia-inducible factors
-
(HIFs). A family of heterodimeric transcription factors that become activated during hypoxia or pseudohypoxia in a cell, and are responsible for potentially altering the expression of hundreds of genes involved in regulating cellular responses to hypoxia.
- Mitochondrial DNA
-
(mtDNA). Circular loops of DNA containing ∼ 16.6 kilobases, located in the matrix of mitochondria. This DNA encodes 13 proteins, ribosomal RNAs and transfer RNAs that are required for a functional oxidative phosphorylation system.
- Cybrids
-
Experimental cells that are formed by fusing a cell lacking mitochondrial DNA with an enucleated cytoplast containing mutant mitochondria.
- Pseudohypoxic activators
-
Stimuli that trigger activation of cellular responses to hypoxia, even though the O2 level in the cell is normal.
- AMP-activated protein kinase
-
(AMPK). A complex consisting of three proteins that has a central role in the regulation of cellular energy production and energy utilization.
Rights and permissions
About this article
Cite this article
Sabharwal, S., Schumacker, P. Mitochondrial ROS in cancer: initiators, amplifiers or an Achilles' heel?. Nat Rev Cancer 14, 709–721 (2014). https://doi.org/10.1038/nrc3803
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrc3803
This article is cited by
-
Role of reactive oxygen species in ultraviolet-induced photodamage of the skin
Cell Division (2024)
-
Oxidative stress promotes cytotoxicity in human cancer cell lines exposed to Escallonia spp. extracts
BMC Complementary Medicine and Therapies (2024)
-
Toxoflavin analog D43 exerts antiproliferative effects on breast cancer by inducing ROS-mediated apoptosis and DNA damage
Scientific Reports (2024)
-
Cuproptosis: mechanisms and links with cancers
Molecular Cancer (2023)
-
Rutaecarpine induces the differentiation of triple-negative breast cancer cells through inhibiting fumarate hydratase
Journal of Translational Medicine (2023)