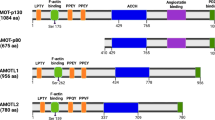
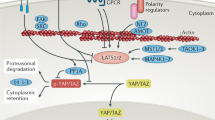
Key Points
-
The Hippo pathway is an evolutionarily conserved regulator of tissue growth.
-
The Hippo pathway controls multiple cellular functions that are central to tumorigenesis, including proliferation and apoptosis.
-
Hippo pathway mutations in mice and flies give rise to tumours.
-
Hippo pathway activity seems to be frequently deregulated in different human cancers but most Hippo pathway genes are not commonly mutated.
-
Molecular events such as sensitivity to the mechanical properties of tumours and crosstalk with other cancer pathways might cause Hippo pathway deregulation in human cancers.
-
Hippo pathway therapeutics and new avenues to modulate pathway activity are beginning to emerge.
Abstract
The Hippo pathway controls organ size in diverse species, whereas pathway deregulation can induce tumours in model organisms and occurs in a broad range of human carcinomas, including lung, colorectal, ovarian and liver cancer. Despite this, somatic or germline mutations in Hippo pathway genes are uncommon, with only the upstream pathway gene neurofibromin 2 (NF2) recognized as a bona fide tumour suppressor gene. In this Review, we appraise the evidence for the Hippo pathway as a cancer signalling network, and discuss cancer-relevant biological functions, potential mechanisms by which Hippo pathway activity is altered in cancer and emerging therapeutic strategies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Tapon, N. et al. salvador promotes both cell cycle exit and apoptosis in Drosophila and is mutated in human cancer cell lines. Cell 110, 467–478 (2002). The discovery of salvador and its functional link to warts outlined the existence of a new growth control pathway in D. melanogaster , known most commonly as the Hippo pathway. This paper also provided the first evidence that the Hippo pathway is perturbed in human cancer.
Justice, R. W., Zilian, O., Woods, D. F., Noll, M. & Bryant, P. J. The Drosophila tumor suppressor gene warts encodes a homolog of human myotonic dystrophy kinase and is required for the control of cell shape and proliferation. Genes Dev. 9, 534–546 (1995).
Xu, T., Wang, W., Zhang, S., Stewart, R. A. & Yu, W. Identifying tumor suppressors in genetic mosaics: the Drosophila lats gene encodes a putative protein kinase. Development 121, 1053–1063 (1995).
Halder, G. & Johnson, R. L. Hippo signaling: growth control and beyond. Development 138, 9–22 (2011).
Harvey, K. & Tapon, N. The Salvador-Warts-Hippo pathway - an emerging tumour-suppressor network. Nature Rev. Cancer 7, 182–191 (2007).
Harvey, K. F., Pfleger, C. M. & Hariharan, I. K. The Drosophila Mst ortholog, hippo, restricts growth and cell proliferation and promotes apoptosis. Cell 114, 457–467 (2003).
Udan, R. S., Kango-Singh, M., Nolo, R., Tao, C. & Halder, G. Hippo promotes proliferation arrest and apoptosis in the Salvador/Warts pathway. Nature Cell Biol. 5, 914–920 (2003).
Pantalacci, S., Tapon, N. & Leopold, P. The Salvador partner Hippo promotes apoptosis and cell-cycle exit in Drosophila. Nature Cell Biol. 5, 921–927 (2003).
Wu, S., Huang, J., Dong, J. & Pan, D. hippo encodes a Ste-20 family protein kinase that restricts cell proliferation and promotes apoptosis in conjunction with salvador and warts. Cell 114, 445–456 (2003).
Kango-Singh, M. et al. Shar-pei mediates cell proliferation arrest during imaginal disc growth in Drosophila. Development 129, 5719–5730 (2002).
Huang, J., Wu, S., Barrera, J., Matthews, K. & Pan, D. The Hippo signaling pathway coordinately regulates cell proliferation and apoptosis by inactivating Yorkie, the Drosophila Homolog of YAP. Cell 122, 421–434 (2005). This paper described the identification of the YKI transcriptional regulator as the crucial downstream target of the D. melanogaster Hippo pathway.
Zhao, B. et al. Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev. 21, 2747–2761 (2007).
Dong, J. et al. Elucidation of a universal size-control mechanism in Drosophila and mammals. Cell 130, 1120–1133 (2007).
Hong, W. & Guan, K. L. The YAP and TAZ transcription co-activators: key downstream effectors of the mammalian Hippo pathway. Semin. Cell Dev. Biol. 23, 785–793 (2012).
Grusche, F. A., Richardson, H. E. & Harvey, K. F. Upstream regulation of the hippo size control pathway. Curr. Biol. 20, R574–R582 (2010).
Cordenonsi, M. et al. The Hippo transducer TAZ confers cancer stem cell-related traits on breast cancer cells. Cell 147, 759–772 (2011).
Grzeschik, N. A., Parsons, L. M., Allott, M. L., Harvey, K. F. & Richardson, H. E. Lgl, aPKC, and Crumbs regulate the Salvador/Warts/Hippo pathway through two distinct mechanisms. Curr. Biol. 20, 573–581 (2010).
Robinson, B. S., Huang, J., Hong, Y. & Moberg, K. H. Crumbs regulates Salvador/Warts/Hippo signaling in Drosophila via the FERM-domain protein expanded. Curr. Biol. 20, 582–590 (2010).
Ling, C. et al. The apical transmembrane protein Crumbs functions as a tumor suppressor that regulates Hippo signaling by binding to Expanded. Proc. Natl Acad. Sci. USA 107, 10532–10537 (2010).
Chen, C. L. et al. The apical-basal cell polarity determinant Crumbs regulates Hippo signaling in Drosophila. Proc. Natl Acad. Sci. USA 107, 15810–15815 (2010).
Varelas, X. et al. The Crumbs complex couples cell density sensing to Hippo-dependent control of the TGF-β-SMAD pathway. Dev. Cell 19, 831–844 (2010).
Zhao, M., Szafranski, P., Hall, C. A. & Goode, S. Basolateral junctions utilize warts signaling to control epithelial-mesenchymal transition and proliferation crucial for migration and invasion of Drosophila ovarian epithelial cells. Genetics 178, 1947–1971 (2008). This paper, together with references 17–21, discovered regulatory links between ABCPs and the Hippo pathway.
Yu, F. X. et al. Regulation of the Hippo-YAP pathway by G-protein-coupled receptor signaling. Cell 150, 780–791 (2012). This report, defining GPCRs as upstream regulators of the Hippo pathway, greatly increased the understanding of how mammalian Hippo pathway activity is controlled.
Bennett, F. C. & Harvey, K. F. Fat cadherin modulates organ size in Drosophila via the Salvador/Warts/Hippo signaling pathway. Curr. Biol. 16, 2101–2110 (2006).
Willecke, M. et al. The fat cadherin acts through the hippo tumor-suppressor pathway to regulate tissue size. Curr. Biol. 16, 2090–2100 (2006).
Silva, E., Tsatskis, Y., Gardano, L., Tapon, N. & McNeill, H. The tumor-suppressor gene fat controls tissue growth upstream of expanded in the hippo signaling pathway. Curr. Biol. 16, 2081–2089 (2006).
Cho, E. et al. Delineation of a Fat tumor suppressor pathway. Nature Genet. 38, 1142–1150 (2006).
Camargo, F. D. et al. YAP1 increases organ size and expands undifferentiated progenitor cells. Curr. Biol. 17, 2054–2060 (2007). This paper, together with references 12 and 13, showed that Hippo pathway signalling and function is conserved between D. melanogaster and mammals, and provided initial evidence that Hippo pathway activity is frequently disrupted in human carcinomas.
Schlegelmilch, K. et al. Yap1 acts downstream of α-catenin to control epidermal proliferation. Cell 144, 782–795 (2011).
Heallen, T. et al. Hippo pathway inhibits Wnt signaling to restrain cardiomyocyte proliferation and heart size. Science 332, 458–461 (2011).
Serrano, M., Lin, A. W., McCurrach, M. E., Beach, D. & Lowe, S. W. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell 88, 593–602 (1997).
Tschop, K. et al. A kinase shRNA screen links LATS2 and the pRB tumor suppressor. Genes Dev. 25, 814–830 (2011).
Aylon, Y. et al. A positive feedback loop between the p53 and Lats2 tumor suppressors prevents tetraploidization. Genes Dev. 20, 2687–2700 (2006).
Overholtzer, M. et al. Transforming properties of YAP, a candidate oncogene on the chromosome 11q22 amplicon. Proc. Natl Acad. Sci. USA 103, 12405–12410 (2006).
Zhao, B. et al. Cell detachment activates the Hippo pathway via cytoskeleton reorganization to induce anoikis. Genes Dev. 26, 54–68 (2012).
Zhang, X. et al. The Hippo pathway transcriptional co-activator, YAP, is an ovarian cancer oncogene. Oncogene 30, 2810–2822 (2011).
Izzo, J. G. et al. Pretherapy nuclear factor-κB status, chemoradiation resistance, and metastatic progression in esophageal carcinoma. Mol. Cancer Ther. 5, 2844–2850 (2006).
Gautam, A. & Bepler, G. Suppression of lung tumor formation by the regulatory subunit of ribonucleotide reductase. Cancer Res. 66, 6497–6502 (2006).
Valent, P. et al. Cancer stem cell definitions and terminology: the devil is in the details. Nature Rev. Cancer 12, 767–775 (2012).
Gatenby, R. A. A change of strategy in the war on cancer. Nature 459, 508–509 (2009).
Morata, G. & Ripoll, P. Minutes: mutants of Drosophila autonomously affecting cell division rate. Dev. Biol. 42, 211–221 (1975).
de Beco, S., Ziosi, M. & Johnston, L. A. New frontiers in cell competition. Dev. Dyn. 241, 831–841 (2012).
Davidson, J. D. et al. An increase in the expression of ribonucleotide reductase large subunit 1 is associated with gemcitabine resistance in non-small cell lung cancer cell lines. Cancer Res. 64, 3761–3766 (2004).
Ramalho-Santos, M. Yoon, S., Matsuzaki, Y., Mulligan, R. C. & Melton, D. A. “Stemness”: transcriptional profiling of embryonic and adult stem cells. Science 298, 597–600 (2002).
Steinhardt, A. A. et al. Expression of Yes-associated protein in common solid tumors. Hum. Pathol. 39, 1582–1589 (2008).
Lian, I. et al. The role of YAP transcription coactivator in regulating stem cell self-renewal and differentiation. Genes Dev. 24, 1106–1118 (2010).
Varelas, X. et al. TAZ controls Smad nucleocytoplasmic shuttling and regulates human embryonic stem-cell self-renewal. Nature Cell Biol. 10, 837–848 (2008).
Hong, J. H. et al. TAZ, a transcriptional modulator of mesenchymal stem cell differentiation. Science 309, 1074–1078 (2005).
Schroeder, M. C. & Halder, G. Regulation of the Hippo pathway by cell architecture and mechanical signals. Semin. Cell Dev. Biol. 23, 803–811 (2012).
Wang, Y. Wnt/Planar cell polarity signaling: a new paradigm for cancer therapy. Mol. Cancer Ther. 8, 2103–2109 (2009).
Martin-Belmonte, F. & Perez-Moreno, M. Epithelial cell polarity, stem cells and cancer. Nature Rev. Cancer 12, 23–38 (2012).
Humbert, P. O. et al. Control of tumourigenesis by the Scribble/Dlg/Lgl polarity module. Oncogene 27, 6888–6907 (2008).
Guilford, P. et al. E-cadherin germline mutations in familial gastric cancer. Nature 392, 402–405 (1998).
Kim, N. G., Koh, E., Chen, X. & Gumbiner, B. M. E-cadherin mediates contact inhibition of proliferation through Hippo signaling-pathway components. Proc. Natl Acad. Sci. USA 108, 11930–11935 (2011).
Holley, R. W. Control of growth of mammalian cells in cell culture. Nature 258, 487–490 (1975).
Lallemand, D., Curto, M., Saotome, I., Giovannini, M. & McClatchey, A. I. NF2 deficiency promotes tumorigenesis and metastasis by destabilizing adherens junctions. Genes Dev. 17, 1090–1100 (2003).
Levental, K. R. et al. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell 139, 891–906 (2009).
Dupont, S. et al. Role of YAP/TAZ in mechanotransduction. Nature 474, 179–183 (2011).
Wada, K., Itoga, K., Okano, T., Yonemura, S. & Sasaki, H. Hippo pathway regulation by cell morphology and stress fibers. Development 138, 3907–3914 (2011).
Sansores-Garcia, L. et al. Modulating F-actin organization induces organ growth by affecting the Hippo pathway. EMBO J. 30, 2325–2335 (2011).
Fernandez, B. G. et al. Actin-Capping Protein and the Hippo pathway regulate F-actin and tissue growth in Drosophila. Development 138, 2337–2346 (2011). References 58–61 described regulatory links between the Hippo pathway and the actin cytoskeleton and suggested that the pathway responds to mechanical stimuli.
Butcher, D. T., Alliston, T. & Weaver, V. M. A tense situation: forcing tumour progression. Nature Rev. Cancer 9, 108–122 (2009).
Simpson, C. D., Anyiwe, K. & Schimmer, A. D. Anoikis resistance and tumor metastasis. Cancer Lett. 272, 177–185 (2008).
McClatchey, A. I. et al. Mice heterozygous for a mutation at the Nf2 tumor suppressor locus develop a range of highly metastatic tumors. Genes Dev. 12, 1121–1133 (1998).
Chen, D. et al. LIFR is a breast cancer metastasis suppressor upstream of the Hippo-YAP pathway and a prognostic marker. Nature Med. 18, 1511–1517 (2012).
Stauffer, J. K., Scarzello, A. J., Jiang, Q. & Wiltrout, R. H. Chronic inflammation, immune escape, and oncogenesis in the liver: a unique neighborhood for novel intersections. Hepatology 56, 1567–1574 (2012).
Staley, B. K. & Irvine, K. D. Warts and Yorkie mediate intestinal regeneration by influencing stem cell proliferation. Curr. Biol. 20, 1580–1587 (2010).
Shaw, R. L. et al. The Hippo pathway regulates intestinal stem cell proliferation during Drosophila adult midgut regeneration. Development 137, 4147–4158 (2010).
Karpowicz, P., Perez, J. & Perrimon, N. The Hippo tumor suppressor pathway regulates intestinal stem cell regeneration. Development 137, 4135–4145 (2010).
Grusche, F. A., Degoutin, J. L., Richardson, H. E. & Harvey, K. F. The Salvador/Warts/Hippo pathway controls regenerative tissue growth in Drosophila melanogaster. Dev. Biol. 350, 255–266 (2011).
Sun, G. & Irvine, K. D. Regulation of Hippo signaling by Jun kinase signaling during compensatory cell proliferation and regeneration, and in neoplastic tumors. Dev. Biol. 350, 139–151 (2011).
Cai, J. et al. The Hippo signaling pathway restricts the oncogenic potential of an intestinal regeneration program. Genes Dev. 24, 2383–2388 (2010).
Hall, C. A. et al. Hippo pathway effector Yap is an ovarian cancer oncogene. Cancer Res. 70, 8517–8525 (2010).
Xu, M. Z. et al. Yes-associated protein is an independent prognostic marker in hepatocellular carcinoma. Cancer 115, 4576–4585 (2009).
Wang, Y. et al. Overexpression of yes-associated protein contributes to progression and poor prognosis of non-small-cell lung cancer. Cancer Sci. 101, 1279–1285 (2010).
Evans, D. G. Neurofibromatosis 2 [Bilateral acoustic neurofibromatosis, central neurofibromatosis, NF2, neurofibromatosis type II]. Genet. Med. 11, 599–610 (2009).
Zender, L. et al. Identification and validation of oncogenes in liver cancer using an integrative oncogenomic approach. Cell 125, 1253–1267 (2006). This paper, and reference 34, presented evidence that YAP1 is an oncogene and is amplified in human tumours.
St John, M. A. et al. Mice deficient of Lats1 develop soft-tissue sarcomas, ovarian tumours and pituitary dysfunction. Nature Genet. 21, 182–186 (1999).
Zhou, D. et al. Mst1 and Mst2 maintain hepatocyte quiescence and suppress hepatocellular carcinoma development through inactivation of the Yap1 oncogene. Cancer Cell 16, 425–438 (2009).
Lu, L. et al. Hippo signaling is a potent in vivo growth and tumor suppressor pathway in the mammalian liver. Proc. Natl Acad. Sci. USA 107, 1437–1442 (2010).
Song, H. et al. Mammalian Mst1 and Mst2 kinases play essential roles in organ size control and tumor suppression. Proc. Natl Acad. Sci. USA 107, 1431–1436 (2010).
Zhou, D. et al. Mst1 and Mst2 protein kinases restrain intestinal stem cell proliferation and colonic tumorigenesis by inhibition of Yes-associated protein (Yap) overabundance. Proc. Natl Acad. Sci. USA 108, e1312–e1320 (2011).
Takahashi, Y. et al. Down-regulation of LATS1 and LATS2 mRNA expression by promoter hypermethylation and its association with biologically aggressive phenotype in human breast cancers. Clin. Cancer Res. 11, 1380–1385 (2005).
Jiang, Z. et al. Promoter hypermethylation-mediated down-regulation of LATS1 and LATS2 in human astrocytoma. Neurosci. Res. 56, 450–458 (2006).
Seidel, C. et al. Frequent hypermethylation of MST1 and MST2 in soft tissue sarcoma. Mol. Carcinog. 46, 865–871 (2007).
Tanas, M. R. et al. Identification of a disease-defining gene fusion in epithelioid hemangioendothelioma. Sci. Transl. Med. 3, 98ra82 (2011).
Errani, C. et al. A novel WWTR1-CAMTA1 gene fusion is a consistent abnormality in epithelioid hemangioendothelioma of different anatomic sites. Genes Chromosomes Cancer 50, 644–653 (2011). References 86 and 87 discovered a chromosomal translocation causing the fusion of the genes encoding TAZ and CAMTA1 as the defining genetic lesion in epithelioid haemangioendothelioma.
Irvine, K. D. Integration of intercellular signaling through the Hippo pathway. Semin. Cell Dev. Biol. 23, 812–817 (2012).
White, B. D., Chien, A. J. & Dawson, D. W. Dysregulation of Wnt/β-catenin signaling in gastrointestinal cancers. Gastroenterology 142, 219–232 (2012).
Bellam, N. & Pasche, B. Tgf-β signaling alterations and colon cancer. Cancer Treat. Res. 155, 85–103 (2010).
Cohen, D. J. Targeting the hedgehog pathway: role in cancer and clinical implications of its inhibition. Hematol. Oncol. Clin. North Am. 26, 565–588 (2012).
Lobry, C., Oh, P. & Aifantis, I. Oncogenic and tumor suppressor functions of Notch in cancer: it's NOTCH what you think. J. Exp. Med. 208, 1931–1935 (2011).
Imajo, M., Miyatake, K., Iimura, A., Miyamoto, A. & Nishida, E. A molecular mechanism that links Hippo signalling to the inhibition of Wnt/β-catenin signalling. EMBO J. 31, 1109–1122 (2012).
Konsavage, W. M. et al. Wnt/β-catenin signaling regulates Yes-associated protein (YAP) gene expression in colorectal carcinoma cells. J. Biol. Chem. 287, 11730–11739 (2012).
Miller, E. et al. Identification of serum-derived sphingosine-1-phosphate as a small molecule regulator of YAP. Chem. Biol. 19, 955–962 (2012).
Lin, S. et al. The absence of LPA2 attenuates tumor formation in an experimental model of colitis-associated cancer. Gastroenterology 136, 1711–1720 (2009).
Onken, M. D. et al. Oncogenic mutations in GNAQ occur early in uveal melanoma. Invest. Ophthalmol. Vis. Sci. 49, 5230–5234 (2008).
Prickett, T. D. et al. Exon capture analysis of G protein-coupled receptors identifies activating mutations in GRM3 in melanoma. Nature Genet. 43, 1119–1126 (2011).
Kan, Z. et al. Diverse somatic mutation patterns and pathway alterations in human cancers. Nature 466, 869–873 (2010).
Van Raamsdonk, C. D. et al. Frequent somatic mutations of GNAQ in uveal melanoma and blue naevi. Nature 457, 599–602 (2009).
Puca, R., Nardinocchi, L., Givol, D. & D'Orazi, G. Regulation of p53 activity by HIPK2: molecular mechanisms and therapeutical implications in human cancer cells. Oncogene 29, 4378–4387 (2010).
Poon, C. L., Zhang, X., Lin, J. I., Manning, S. A. & Harvey, K. F. Homeodomain-interacting protein kinase regulates hippo pathway-dependent tissue growth. Curr. Biol. 22, 1587–1594 (2012).
Chen, J. & Verheyen, E. M. Homeodomain-interacting protein kinase regulates yorkie activity to promote tissue growth. Curr. Biol. 22, 1582–1586 (2012).
Zhang, N. et al. The Merlin/NF2 tumor suppressor functions through the YAP oncoprotein to regulate tissue homeostasis in mammals. Dev. Cell 19, 27–38 (2010). This publication proved that YAP is a key driver of tumorigenesis and tissue overgrowth caused by loss of Nf2 in the murine liver.
Liu-Chittenden, Y. et al. Genetic and pharmacological disruption of the TEAD-YAP complex suppresses the oncogenic activity of YAP. Genes Dev. 26, 1300–1305 (2012). Porphyrin compounds were identified as potential antitumour agents on the basis of their ability to disrupt the interaction of the YAP oncoprotein with the TEAD1–4 transcription factors.
Michels, S. & Schmidt-Erfurth, U. Photodynamic therapy with verteporfin: a new treatment in ophthalmology. Semin. Ophthalmol. 16, 201–206 (2001).
Agostinis, P. et al. Photodynamic therapy of cancer: an update. CA Cancer J. Clin. 61, 250–281 (2011).
Sjogren, B. Regulator of G protein signaling proteins as drug targets: current state and future possibilities. Adv. Pharmacol. 62, 315–347 (2011).
Bao, Y. et al. A cell-based assay to screen stimulators of the Hippo pathway reveals the inhibitory effect of dobutamine on the YAP-dependent gene transcription. J. Biochem. 150, 199–208 (2011).
Murph, M. & Mills, G. B. Targeting the lipids LPA and S1P and their signalling pathways to inhibit tumour progression. Expert Rev. Mol. Med. 9, 1–18 (2007).
Fleming, J. K., Wojciak, J. M., Campbell, M. A. & Huxford, T. Biochemical and structural characterization of lysophosphatidic Acid binding by a humanized monoclonal antibody. J. Mol. Biol. 408, 462–476 (2011).
Wojciak, J. M. et al. The crystal structure of sphingosine-1-phosphate in complex with a Fab fragment reveals metal bridging of an antibody and its antigen. Proc. Natl Acad. Sci. USA 106, 17717–17722 (2009).
Ponnusamy, S. et al. Communication between host organism and cancer cells is transduced by systemic sphingosine kinase 1/sphingosine 1-phosphate signalling to regulate tumour metastasis. EMBO Mol. Med. 4, 761–775 (2012).
Clair, T. et al. Autotaxin hydrolyzes sphingosylphosphorylcholine to produce the regulator of migration, sphingosine-1-phosphate. Cancer Res. 63, 5446–5453 (2003).
Umezu-Goto, M. et al. Autotaxin has lysophospholipase D activity leading to tumor cell growth and motility by lysophosphatidic acid production. J. Cell Biol. 158, 227–233 (2002).
Stracke, M. L. et al. Identification, purification, and partial sequence analysis of autotaxin, a novel motility-stimulating protein. J. Biol. Chem. 267, 2524–2529 (1992).
Tanaka, M. et al. Autotaxin stabilizes blood vessels and is required for embryonic vasculature by producing lysophosphatidic acid. J. Biol. Chem. 281, 25822–25830 (2006).
Xia, P. et al. An oncogenic role of sphingosine kinase. Curr. Biol. 10, 1527–1530 (2000).
Van Brocklyn, J. R. et al. Sphingosine kinase-1 expression correlates with poor survival of patients with glioblastoma multiforme: roles of sphingosine kinase isoforms in growth of glioblastoma cell lines. J. Neuropathol. Exp. Neurol. 64, 695–705 (2005).
de Souza, P. L. et al. Phase I and pharmacokinetic study of weekly NV06 (Phenoxodiol), a novel isoflav-3-ene, in patients with advanced cancer. Cancer Chemother. Pharmacol. 58, 427–433 (2006).
Kelly, M. G. et al. Phase II evaluation of phenoxodiol in combination with cisplatin or paclitaxel in women with platinum/taxane-refractory/resistant epithelial ovarian, fallopian tube, or primary peritoneal cancers. Int. J. Gynecol. Cancer 21, 633–639 (2011).
Bertini, E., Oka, T., Sudol, M., Strano, S. & Blandino, G. YAP: at the crossroad between transformation and tumor suppression. Cell Cycle 8, 49–57 (2009).
Louvi, A. & Artavanis-Tsakonas, S. Notch and disease: a growing field. Semin. Cell Dev. Biol. 23, 473–480 (2012).
Samanta, D. & Datta, P. K. Alterations in the Smad pathway in human cancers. Front. Biosci. 17, 1281–1293 (2012).
Barry, E. R. et al. Restriction of intestinal stem cell expansion and the regenerative response by YAP. Nature 493, 106–110 (2013).
Altomare, D. A. et al. A mouse model recapitulating molecular features of human mesothelioma. Cancer Res. 65, 8090–8095 (2005).
Giovannini, M. et al. Conditional biallelic Nf2 mutation in the mouse promotes manifestations of human neurofibromatosis type 2. Genes Dev. 14, 1617–1630 (2000).
Kalamarides, M. et al. Nf2 gene inactivation in arachnoidal cells is rate-limiting for meningioma development in the mouse. Genes Dev. 16, 1060–1065 (2002).
Morris, Z. S. & McClatchey, A. I. Aberrant epithelial morphology and persistent epidermal growth factor receptor signaling in a mouse model of renal carcinoma. Proc. Natl Acad. Sci. USA 106, 9767–9772 (2009).
Benhamouche, S. et al. Nf2/Merlin controls progenitor homeostasis and tumorigenesis in the liver. Genes Dev. 24, 1718–1730 (2010).
Kim, T. S. et al. Mammalian sterile 20-like kinase 1 (Mst1) suppresses lymphoma development by promoting faithful chromosome segregation. Cancer Res. 72, 5386–5395 (2012).
Lee, K. P. et al. The Hippo-Salvador pathway restrains hepatic oval cell proliferation, liver size, and liver tumorigenesis. Proc. Natl Acad. Sci. USA 107, 8248–8253 (2010).
Nishio, M. et al. Cancer susceptibility and embryonic lethality in Mob1a/1b double-mutant mice. J. Clin. Invest. 122, 4505–4518 (2012).
Varelas, X. et al. The Hippo pathway regulates Wnt/β-catenin signaling. Dev. Cell 18, 579–591 (2010). This paper, together with reference 30, discovered mechanisms of crosstalk between the Hippo and WNT pathways.
Alarcon, C. et al. Nuclear CDKs drive Smad transcriptional activation and turnover in BMP and TGF-β pathways. Cell 139, 757–769 (2009).
Fernandez, L. A. et al. YAP1 is amplified and up-regulated in hedgehog-associated medulloblastomas and mediates Sonic hedgehog-driven neural precursor proliferation. Genes Dev. 23, 2729–2741 (2009).
Tumaneng, K. et al. YAP mediates crosstalk between the Hippo and PI(3)K-TOR pathways by suppressing PTEN via miR-29. Nature Cell Biol. 14, 1322–1329 (2012).
Polesello, C. & Tapon, N. Salvador-warts-hippo signaling promotes Drosophila posterior follicle cell maturation downstream of notch. Curr. Biol. 17, 1864–1870 (2007).
Acknowledgements
K.F.H. is a Sylvia and Charles Viertel Senior Medical Research Fellow. X.Z. is a Cure Cancer Australia and National Breast Cancer Foundation fellow. D.M.T is an Australian National Health and Medical Research Council (NHMRC) Senior Research Fellow.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Supplementary information
Supplementary information S1 (table)
Detailed somatic mutations in Hippo pathway genes in human cancer (PDF 361 kb)
Glossary
- 14-3-3 binding sites
-
Peptide sequences that bind to 14-3-3 proteins when phosphorylated. 14-3-3 proteins often function as adaptor proteins or subcellular localizers of their protein substrates.
- Anoikis
-
Apoptosis resulting from inappropriate attachment of cells to a substrate.
- Pluripotency
-
The capability of cells to give rise to multiple lineages.
- Non-canonical WNT signalling
-
WNT signalling that is independent of β-catenin.
- Adherens junctions
-
Intercellular junctions important for epithelial cell–cell adhesion.
- Tight junctions
-
Intercellular junctions that form at the apical regions of epithelial cells.
- Driver mutations
-
Mutations that are actively involved in tumour formation.
- Passenger mutations
-
Mutations that are present in cancers but that do not promote tumour formation.
- Pathognomonic
-
Distinctive for a particular disease.
- Epithelioid haemangioendothelioma
-
A rare vascular tumour characterized by TAZ–CAMTA1 gene fusions.
- Photocoagulation therapy
-
Light-based method used especially for treating retinal tears.
- Photodynamic therapy
-
Activation of photosensitive compounds by light.
Rights and permissions
About this article
Cite this article
Harvey, K., Zhang, X. & Thomas, D. The Hippo pathway and human cancer. Nat Rev Cancer 13, 246–257 (2013). https://doi.org/10.1038/nrc3458
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrc3458
This article is cited by
-
FTO attenuates the cytotoxicity of cisplatin in KGN granulosa cell-like tumour cells by regulating the Hippo/YAP1 signalling pathway
Journal of Ovarian Research (2024)
-
Transcriptomics analysis revealed that TAZ regulates the proliferation of KIRC cells through mitophagy
BMC Cancer (2024)
-
VPS35 promotes gastric cancer progression through integrin/FAK/SRC signalling-mediated IL-6/STAT3 pathway activation in a YAP-dependent manner
Oncogene (2024)
-
Alternative Wnt-signaling axis leads to a break of oncogene-induced senescence
Cell Death & Disease (2024)
-
Targeting metastasis-initiating cancer stem cells in gastric cancer with leukaemia inhibitory factor
Cell Death Discovery (2024)