Abstract
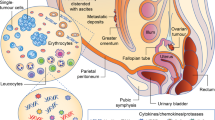
Malignant ascites presents a considerable clinical challenge to the management of ovarian cancer, but also provides a wealth of opportunities for translational research. The accessibility of ascitic fluid and its cellular components make it an excellent source of tumour tissue for the investigation of prognostic and predictive biomarkers, pharmacodynamic markers and for molecular profiling analysis. In this Opinion article, we discuss recent advances in our understanding of its pathophysiology, the development of new methods to characterize its molecular features and how these findings can be used to improve the treatment of malignant ascites, particularly in the context of ovarian cancer.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Runyon, B. A. Care of patients with ascites. N. Engl. J. Med. 330, 337–342 (1994).
Parsons, S. L., Lang, M. W. & Steele, R. J. Malignant ascites: a 2-year review from a teaching hospital. Eur. J. Surg. Oncol. 22, 237–239 (1996).
Ayantunde, A. & Parsons, S. Pattern and prognostic factors in patients with malignant ascites: a retrospective study. Ann. Oncol. 18, 945–949 (2007).
Garrison, R. N., Kaelin, L. D., Galloway, R. H. & Heuser, L. S. Malignant ascites. Clinical and experimental observations. Ann. Surg. 203, 644–651 (1986).
Howlader, N. et al. SEER Cancer Statistics Review, 1975–2009 (Vintage 2009 Populations). National Cancer Institute [online], (2012).
Lopez, R. I. et al. Prognostic factor analysis, for patients with no evidence of disease after initial chemotherapy for advanced epithelial ovarian carcinoma. Int. J. Gynecol. Cancer 6, 8–14 (1996).
Tan, D. S., Agarwal, R. & Kaye, S. B. Mechanisms of transcoelomic metastasis in ovarian cancer. Lancet Oncol. 7, 925–934 (2006).
Bagnato, A. & Rosano, L. Epithelial-mesenchymal transition in ovarian cancer progression: a crucial role for the endothelin axis. Cells Tissues Organs 185, 85–94 (2007).
Auersperg, N., Wong, A. S., Choi, K. C., Kang, S. K. & Leung, P. C. Ovarian surface epithelium: biology, endocrinology, and pathology. Endocr. Rev. 22, 255–288 (2001).
Gardner, M. J., Catterall, J. B., Jones, L. M. & Turner, G. A. Human ovarian tumour cells can bind hyaluronic acid via membrane CD44: a possible step in peritoneal metastasis. Clin. Exp. Metastasis 14, 325–334 (1996).
Strobel, T., Swanson, L. & Cannistra, S. A. In vivo inhibition of CD44 limits intra-abdominal spread of a human ovarian cancer xenograft in nude mice: a novel role for CD44 in the process of peritoneal implantation. Cancer Res. 57, 1228–1232 (1997).
Wagner, B. J. et al. Simvastatin reduces tumor cell adhesion to human peritoneal mesothelial cells by decreased expression of VCAM-1 and β1 integrin. Int. J. Oncol. 39, 1593–1600 (2011).
Rump, A. et al. Binding of ovarian cancer antigen CA125/MUC16 to mesothelin mediates cell adhesion. J. Biol. Chem. 279, 9190–9198 (2004).
Gubbels, J. A. et al. Mesothelin-MUC16 binding is a high affinity, N-glycan dependent interaction that facilitates peritoneal metastasis of ovarian tumors. Mol. Cancer 566, 50 (2006).
Renkin, E. M. Some consequences of capillary permeability to macromolecules: Starling's hypothesis reconsidered. Am. J. Physiol. 250, H706–710 (1986).
Mutsaers, S. E. The mesothelial cell. Int. J. Biochem. Cell Biol. 36, 9–16 (2004).
Mutsaers, S. E. Mesothelial cells: their structure, function and role in serosal repair. Respirology 7, 171–191 (2002).
von Recklinghausen, F. T. Zur fettre sorption. Arch. Pathol. Anat. Physiol. 2666, 172 (1863).
Sodek, K. L., Murphy, K. J., Brown, T. J. & Ringuette, M. J. Cell-cell and cell-matrix dynamics in intraperitoneal cancer metastasis. Cancer Metastasis Rev. 31, 397–414 (2012).
Holm-Nielsen, P. Pathogenesis of ascites in peritoneal carcinomatosis. Acta Pathol. Microbiol. Scand. 33, 10–21 (1953).
Feldman, G. B., Knapp, R. C., Order, S. E. & Hellman, S. The role of lymphatic obstruction in the formation of ascites in a murine ovarian carcinoma. Cancer Res. 32, 1663–1666 (1972).
Nagy, J. A., Herzberg, K. T., Dvorak, J. M. & Dvorak, H. F. Pathogenesis of malignant ascites formation: initiating events that lead to fluid accumulation. Cancer Res. 53, 2631–2643 (1993).
Garrison, R. N., Galloway, R. H. & Heuser, L. S. Mechanisms of malignant ascites production. J. Surg. Res. 42, 126–132 (1987).
Hirabayashi, K. & Graham, J. Genesis of ascites in ovarian cancer. Am. J. Obstet. Gynecol. 106, 492–497 (1970).
Senger, D. R. et al. Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science 219, 983–985 (1983).
Neufeld, G., Cohen, T., Gengrinovitch, S. & Poltorak, Z. Vascular endothelial growth factor (VEGF) and its receptors. FASEB J. 13, 9–22 (1999).
Geva, E. & Jaffe, R. B. Role of vascular endothelial growth factor in ovarian physiology and pathology. Fertil. Steril. 74, 429–438 (2000).
Zebrowski, B. K. et al. Markedly elevated levels of vascular endothelial growth factor in malignant ascites. Ann. Surg. Oncol. 6, 373–378 (1999).
Barton, D. P. et al. Angiogenic protein expression in advanced epithelial ovarian cancer. Clin. Cancer Res. 3, 1579–1586 (1997).
Kassim, S. K. et al. Vascular endothelial growth factor and interleukin-8 are associated with poor prognosis in epithelial ovarian cancer patients. Clin. Biochem. 37, 363–369 (2004).
Paley, P. J. et al. Vascular endothelial growth factor expression in early stage ovarian carcinoma. Cancer 80, 98–106 (1997).
Bamias, A. et al. Correlation of NK T-like CD3+CD56+ cells and CD4+CD25+(hi) regulatory T cells with VEGF and TNFα in ascites from advanced ovarian cancer: association with platinum resistance and prognosis in patients receiving first-line, platinum-based chemotherapy. Gynecol. Oncol. 108, 421–427 (2008).
Santin, A. D. et al. Secretion of vascular endothelial growth factor in ovarian cancer. Eur. J. Gynaecol. Oncol. 20, 177–181 (1999).
Schumacher, J. J. et al. Modulation of angiogenic phenotype alters tumorigenicity in rat ovarian epithelial cells. Cancer Res. 67, 3683–3690 (2007).
Byrne, A. T. et al. Vascular endothelial growth factor-trap decreases tumor burden, inhibits ascites, and causes dramatic vascular remodeling in an ovarian cancer model. Clin. Cancer Res. 9, 5721–5728 (2003).
Mesiano, S., Ferrara, N. & Jaffe, R. B. Role of vascular endothelial growth factor in ovarian cancer: inhibition of ascites formation by immunoneutralization. Am. J. Pathol. 153, 1249–1256 (1998).
Yukita, A., Asano, M., Okamoto, T., Mizutani, S. & Suzuki, H. Suppression of ascites formation and re-accumulation associated with human ovarian cancer by an anti-VPF monoclonal antibody in vivo. Anticancer Res. 20, 155–160 (2000).
Herr, D. et al. VEGF induces ascites in ovarian cancer patients via increasing peritoneal permeability by downregulation of Claudin 5. Gynecol. Oncol. 127, 210–216 (2012).
Rodewald, M. et al. Regulation of tight junction proteins occludin and claudin 5 in the primate ovary during the ovulatory cycle and after inhibition of vascular endothelial growth factor. Mol. Hum. Reprod. 13, 781–789 (2007).
Saitou, M. et al. Complex phenotype of mice lacking occludin, a component of tight junction strands. Mol. Biol. Cell 11, 4131–4142 (2000).
Dejana, E. Endothelial cell-cell junctions: happy together. Nature Rev. Mol. Cell Biol. 5, 261–270 (2004).
Takahashi, A., Kondoh, M., Kodaka, M. & Yagi, K. Peptides as tight junction modulators. Curr. Pharm. Des. 17, 2699–2703 (2011).
Nitta, T. et al. Size-selective loosening of the blood-brain barrier in claudin-5-deficient mice. J. Cell Biol. 161, 653–660 (2003).
Dvorak, H. F., Nagy, J. A., Feng, D., Brown, L. F. & Dvorak, A. M. Vascular permeability factor/vascular endothelial growth factor and the significance of microvascular hyperpermeability in angiogenesis. Curr. Top. Microbiol. Immunol. 237, 97–132 (1999).
Esser, S., Lampugnani, M. G., Corada, M., Dejana, E. & Risau, W. Vascular endothelial growth factor induces VE-cadherin tyrosine phosphorylation in endothelial cells. J. Cell Sci. 111, 1853–1865 (1998).
Horiuchi, A. et al. Hypoxia-induced changes in the expression of VEGF, HIF-1 α and cell cycle-related molecules in ovarian cancer cells. Anticancer Res. 22, 2697–2702 (2002).
Hu, Y. L. et al. Lysophosphatidic acid induction of vascular endothelial growth factor expression in human ovarian cancer cells. J. Natl Cancer Inst. 93, 762–768 (2001).
Kulbe, H. et al. The inflammatory cytokine tumor necrosis factor-α generates an autocrine tumor-promoting network in epithelial ovarian cancer cells. Cancer Res. 67, 585–592 (2007).
Salani, D. et al. Role of endothelin-1 in neovascularization of ovarian carcinoma. Am. J. Pathol. 157, 1537–1547 (2000).
Gupta, R. A. et al. Cyclooxygenase-1 is overexpressed and promotes angiogenic growth factor production in ovarian cancer. Cancer Res. 63, 906–911 (2003).
Stadlmann, S. et al. Ovarian carcinoma cells and IL-1β-activated human peritoneal mesothelial cells are possible sources of vascular endothelial growth factor in inflammatory and malignant peritoneal effusions. Gynecol. Oncol. 97, 784–789 (2005).
Belotti, D. et al. Matrix metalloproteinases (MMP9 and MMP2) induce the release of vascular endothelial growth factor (VEGF) by ovarian carcinoma cells: implications for ascites formation. Cancer Res. 63, 5224–5229 (2003).
Chen, Y., Gou, X., Ke, X., Cui, H. & Chen, Z. Human tumor cells induce angiogenesis through positive feedback between CD147 and insulin-like growth factor-I. PLoS ONE 766, e40965 (2012).
Liu, L. Z. et al. Reactive oxygen species regulate epidermal growth factor-induced vascular endothelial growth factor and hypoxia-inducible factor-1α expression through activation of AKT and P70S6K1 in human ovarian cancer cells. Free Radic. Biol. Med. 41, 1521–1533 (2006).
Matei, D. et al. PDGF BB induces VEGF secretion in ovarian cancer. Cancer Biol. Ther. 6, 1951–1959 (2007).
Liao, S. et al. TGF-β blockade controls ascites by preventing abnormalization of lymphatic vessels in orthotopic human ovarian carcinoma models. Clin. Cancer Res. 17, 1415–1424 (2011).
Mills, G. B., May, C., McGill, M., Roifman, C. M. & Mellors, A. A. Putative new growth factor in ascitic fluid from ovarian cancer patients: identification, characterization, and mechanism of action. Cancer Res. 48, 1066–1071 (1988).
Fang, X. et al. Mechanisms for lysophosphatidic acid-induced cytokine production in ovarian cancer cells. J. Biol. Chem. 279, 9653–9661 (2004).
Murph, M. M. et al. Lysophosphatidic acid-induced transcriptional profile represents serous epithelial ovarian carcinoma and worsened prognosis. PLoS ONE 4, e5583 (2009).
Jankowski, M. Autotaxin: its role in biology of melanoma cells and as a pharmacological target. Enzyme Res. 19, 48–57 (2011).
Bast, R. C. Jr, Hennessy, B. & Mills, G. B. The biology of ovarian cancer: new opportunities for translation. Nature Rev. Cancer 9, 415–428 (2009).
Mills, G. B. & Moolenaar, W. H. The emerging role of lysophosphatidic acid in cancer. Nature Rev. Cancer 3, 582–591 (2003).
Abrahams, V. M. et al. Epithelial ovarian cancer cells secrete functional Fas ligand. Cancer Res. 63, 5573–5581 (2003).
Bjorge, L. et al. Ascitic complement system in ovarian cancer. Br. J. Cancer 92, 895–905 (2005).
Webb, T. J. et al. Molecular identification of GD3 as a suppressor of the innate immune response in ovarian cancer. Cancer Res. 72, 3744–3752 (2012).
Milliken, D., Scotton, C., Raju, S., Balkwill, F. & Wilson, J. Analysis of chemokines and chemokine receptor expression in ovarian cancer ascites. Clin. Cancer Res. 8, 1108–1114 (2002).
Guo, Y. et al. Effects of siltuximab on the IL-6-induced signaling pathway in ovarian cancer. Clin. Cancer Res. 16, 5759–5769 (2010).
Matte, I., Lane, D., Laplante, C., Rancourt, C. & Piche, A. Profiling of cytokines in human epithelial ovarian cancer ascites. Am. J. Cancer Res. 2, 566–580 (2012).
Penson, R. T. et al. Cytokines IL-1β, IL-2, IL-6, IL-8, MCP-1, GM-CSF and TNFα in patients with epithelial ovarian cancer and their relationship to treatment with paclitaxel. Int. J. Gynecol. Cancer 10, 33–41 (2000).
Kryczek, I., Grybos, M., Karabon, L., Klimczak, A. & Lange, A. IL-6 production in ovarian carcinoma is associated with histiotype and biological characteristics of the tumour and influences local immunity. Br. J. Cancer 82, 621–628 (2000).
Huang, S., Robinson, J. B., DeGuzman, A., Bucana, C. D. & Fidler, I. J. Blockade of nuclear factor-κB signaling inhibits angiogenesis and tumorigenicity of human ovarian cancer cells by suppressing expression of vascular endothelial growth factor and interleukin 8. Cancer Res. 60, 5334–5339 (2000).
Yoneda, J. et al. Expression of angiogenesis-related genes and progression of human ovarian carcinomas in nude mice. J. Natl Cancer Institute 90, 447–454 (1998).
Obata, N., Tamakoshi, K., Shibata, K., Kikkawa, F. & Tomoda, Y. Effects of interleukin-6 on in vitro cell attachment, migration and invasion of human carcinoma. Anticancer Res. 17, 337–342 (1997).
Woolery, K. T. & Kruk, P. A. Ovarian epithelial-stromal interactions: role of interleukins 1 and 6. Obstet. Gynecol. Int. 35, 84–93 (2011).
Nilsson, M. B., Langley, R. R. & Fidler, I. J. Interleukin-6, secreted by human ovarian carcinoma cells, is a potent proangiogenic cytokine. Cancer Res. 65, 10794–10800 (2005).
Alberti, C. et al. Ligand-dependent EGFR activation induces the co-expression of IL-6 and PAI-1 via the NFkB pathway in advanced-stage epithelial ovarian cancer. Oncogene 31, 4139–4149 (2011).
Lane, D., Matte, I., Rancourt, C. & Piche, A. Prognostic significance of IL-6 and IL-8 ascites levels in ovarian cancer patients. BMC Cancer 1166, 210 (2011).
Naldini, A. et al. Identification of thrombin-like activity in ovarian cancer associated ascites and modulation of multiple cytokine networks. Thromb. Haemost. 106, 705–711 (2011).
Gubbels, J. A. et al. MUC16 provides immune protection by inhibiting synapse formation between NK and ovarian tumor cells. Mol. Cancer 966, 11 (2010).
Sica, A., Saccani, A. & Mantovani, A. Tumor-associated macrophages: a molecular perspective. Int. Immunopharmacol. 2, 1045–1054 (2002).
Hagemann, T. et al. Ovarian cancer cells polarize macrophages toward a tumor-associated phenotype. J. Immunol. 176, 5023–5032 (2006).
Balkwill, F. Cancer and the chemokine network. Nature Rev. Cancer 4, 540–550 (2004).
Wang, E. et al. Peritoneal and subperitoneal stroma may facilitate regional spread of ovarian cancer. Clin. Cancer Res. 11, 113–122 (2005).
Lin, Y. G. et al. EphA2 overexpression is associated with angiogenesis in ovarian cancer. Cancer 109, 332–340 (2007).
Niu, G. et al. Constitutive Stat3 activity up-regulates VEGF expression and tumor angiogenesis. Oncogene 21, 2000–2008 (2002).
Rosen, D. G. et al. The role of constitutively active signal transducer and activator of transcription 3 in ovarian tumorigenesis and prognosis. Cancer 107, 2730–2740 (2006).
Lane, D., Robert, V., Grondin, R., Rancourt, C. & Piche, A. Malignant ascites protect against TRAIL-induced apoptosis by activating the PI3K/Akt pathway in human ovarian carcinoma cells. Int. J. Cancer 121, 1227–1237 (2007).
Lane, D., Goncharenko-Khaider, N., Rancourt, C. & Piche, A. Ovarian cancer ascites protects from TRAIL-induced cell death through αvβ5 integrin-mediated focal adhesion kinase and Akt activation. Oncogene 29, 3519–3531 (2010).
Goncharenko-Khaider, N., Matte, I., Lane, D., Rancourt, C. & Piche, A. Ovarian cancer ascites increase Mcl-1 expression in tumor cells through ERK1/2-Elk-1 signaling to attenuate TRAIL-induced apoptosis. Mol. Cancer 1166, 84 (2012).
Peart, T. M., Correa, R. J., Valdes, Y. R., Dimattia, G. E. & Shepherd, T. G. BMP signalling controls the malignant potential of ascites-derived human epithelial ovarian cancer spheroids via AKT kinase activation. Clin. Exp. Metastasis 29, 293–313 (2012).
Bookman, M. A. et al. Evaluation of new platinum-based treatment regimens in advanced-stage ovarian cancer: a Phase III Trial of the Gynecologic Cancer Intergroup. J. Clin. Oncol. 27, 1419–1425 (2009).
De Placido, S. et al. Topotecan compared with no therapy after response to surgery and carboplatin/paclitaxel in patients with ovarian cancer: Multicenter Italian Trials in Ovarian Cancer (MITO-1) randomized study. J. Clin. Oncol. 22, 2635–2642 (2004).
du Bois, A. et al. Addition of epirubicin as a third drug to carboplatin-paclitaxel in first-line treatment of advanced ovarian cancer: a prospectively randomized gynecologic cancer intergroup trial by the Arbeitsgemeinschaft Gynaekologische Onkologie Ovarian Cancer Study Group and the Groupe d'Investigateurs Nationaux pour l'Etude des Cancers Ovariens. J. Clin. Oncol. 24, 1127–1135 (2006).
Perren, T. J. et al. A phase 3 trial of bevacizumab in ovarian cancer. New Engl. J. Med. 365, 2484–2496 (2011).
Burger, R. A. et al. Incorporation of bevacizumab in the primary treatment of ovarian cancer. New Engl. J. Med. 365, 2473–2483 (2011).
Kristensen, G. P. et al. Result of interim analysis of overall survival in the GCIG ICON7 phase III randomized trial of bevacizumab in women with newly diagnosed ovarian cancer. J. Clin. Oncol. Abstr. 29, LBA5006 (2011).
Aghajanian, C. et al. OCEANS: a randomized, double-blind, placebo-controlled phase III trial of chemotherapy with or without bevacizumab in patients with platinum-sensitive recurrent epithelial ovarian, primary peritoneal, or fallopian tube cancer. J. Clin. Oncol. 30, 2039–2045 (2012).
Pujade-Lauraine, E. et al. AURELIA: a randomized phase III trial evaluating bevacizumab (BEV) plus chemotherapy (CT) for platinum (PT)-resistant recurrent ovarian cancer (OC). J. Clin. Oncol. Abstr. 30, LBA5002 (2012).
Sennino, B. & McDonald, D. M. Controlling escape from angiogenesis inhibitors. Nature Rev. Cancer 12, 699–709 (2012).
Runyon, B. A. Management of adult patients with ascites due to cirrhosis: an update. Hepatology 49, 2087–2107 (2009).
Lee, C. W., Bociek, G. & Faught, W. A survey of practice in management of malignant ascites. J. Pain Symptom Manage. 16, 96–101 (1998).
Becker, G., Galandi, D. & Blum, H. E. Malignant ascites: systematic review and guideline for treatment. Eur. J. Cancer 42, 589–597 (2006).
Pockros, P. J., Esrason, K. T., Nguyen, C., Duque, J. & Woods, S. Mobilization of malignant ascites with diuretics is dependent on ascitic fluid characteristics. Gastroenterology 103, 1302–1306 (1992).
Cavazzoni, E., Bugiantella, W., Graziosi, L., Franceschini, M. S. & Donini, A. Malignant ascites: pathophysiology and treatment. Int. J. Clin. Oncol. 31 Mar 2012 (doi:10.1007/s10147-012-0396-6).
Kalambokis, G. et al. Renal effects of treatment with diuretics, octreotide or both, in non-azotemic cirrhotic patients with ascites. Nephrol. Dial. Transplant. 20, 1623–1629 (2005).
Jatoi, A. et al. A pilot study of long-acting octreotide for symptomatic malignant ascites. Oncology 82, 315–320 (2012).
Dedrick, R. L., Myers, C. E., Bungay, P. M. & DeVita, V. T. Jr. Pharmacokinetic rationale for peritoneal drug administration in the treatment of ovarian cancer. Cancer Treat. Rep. 62, 1–11 (1978).
Armstrong, D. K. et al. Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N. Engl. J. Med. 354, 34–43 (2006).
Ostrowski, M. J. An assessment of the long-term results of controlling the reaccumulation of malignant effusions using intracavity bleomycin. Cancer 57, 721–727 (1986).
Maiche, A. G. Management of peritoneal effusions with intracavitary mitoxantrone or bleomycin. Anticancer Drugs 5, 305–308 (1994).
Link, K. H. et al. Intraperitoneal chemotherapy with mitoxantrone in malignant ascites. Surg. Oncol. Clin. N. Am. 12, 865–872 (2003).
Schilsky, R. L. et al. Phase I clinical and pharmacologic study of intraperitoneal cisplatin and fluorouracil in patients with advanced intraabdominal cancer. J. Clin. Oncol. 8, 2054–2061 (1990).
Mackey, J. R., Wood, L., Nabholtz, J., Jensen, J. & Venner, P. A phase II trial of triamcinolone hexacetanide for symptomatic recurrent malignant ascites. J. Pain Symptom Manage. 19, 193–199 (2000).
Stuart, G. C., Nation, J. G., Snider, D. D. & Thunberg, P. Intraperitoneal interferon in the management of malignant ascites. Cancer 71, 2027–2030 (1993).
Rath, U. et al. Effect of intraperitoneal recombinant human tumour necrosis factor α on malignant ascites. Eur. J. Cancer 27, 121–125 (1991).
Heiss, M. M. et al. The trifunctional antibody catumaxomab for the treatment of malignant ascites due to epithelial cancer: results of a prospective randomized phase II/III trial. Int. J. Cancer 127, 2209–2221 (2010).
Ott, M. G. et al. Humoral response to catumaxomab correlates with clinical outcome: results of the pivotal phase II/III study in patients with malignant ascites. Int. J. Cancer 130, 2195–2203 (2012).
Beattie, G. J. & Smyth, J. F. Phase I study of intraperitoneal metalloproteinase inhibitor BB94 in patients with malignant ascites. Clin. Cancer Res. 4, 1899–1902 (1998).
Numnum, T. M., Rocconi, R. P., Whitworth, J. & Barnes, M. N. The use of bevacizumab to palliate symptomatic ascites in patients with refractory ovarian carcinoma. Gynecol. Oncol. 102, 425–428 (2006).
El-Shami, K., Elsaid, A. & El-Kerm, Y. Open-label safety and efficacy pilot trial of intraperitoneal bevacizumab as palliative treatment in refractory malignant ascites. J. Clin. Oncol. 25, 9043 (2007).
Hamilton, C. A. et al. Intraperitoneal bevacizumab for the palliation of malignant ascites in refractory ovarian cancer. Gynecol. Oncol. 111, 530–532 (2008).
Bellati, F. et al. Complete remission of ovarian cancer induced intractable malignant ascites with intraperitoneal bevacizumab. Immunological observations and a literature review. Invest. New Drugs 28, 887–894 (2010).
Holash, J. et al. VEGF-Trap: a VEGF blocker with potent antitumor effects. Proc. Natl Acad. Sci. 99, 11393–11398 (2002).
Colombo, N. et al. A phase II study of aflibercept in patients with advanced epithelial ovarian cancer and symptomatic malignant ascites. Gynecol. Oncol. 125, 42–47 (2012).
Gotlieb, W. H. et al. Intravenous aflibercept for treatment of recurrent symptomatic malignant ascites in patients with advanced ovarian cancer: a phase 2, randomised, double-blind, placebo-controlled study. Lancet Oncol. 13, 154–162 (2012).
LeVeen, H. H. et al. Peritoneo-venous shunting for ascites. Ann. Surg. 180, 580–591 (1974).
Mamada, Y. et al. Peritoneovenous shunts for palliation of malignant ascites. J. Nippon Med. Sch. 74, 355–358 (2007).
White, M. A., Agle, S. C., Padia, R. K. & Zervos, E. E. Denver peritoneovenous shunts for the management of malignant ascites: a review of the literature in the post LeVeen Era. Am. Surg. 77, 1070–1075 (2011).
Saiz-Mendiguren, R. et al. Permanent tunneled drainage for malignant ascites: initial experience with the PleurX® catheter. Radiologia 52, 541–545 (2010).
Fleming, N. D., Alvarez-Secord, A., Von Gruenigen, V., Miller, M. J. & Abernethy, A. P. Indwelling catheters for the management of refractory malignant ascites: a systematic literature overview and retrospective chart review. J. Pain Symptom Manage. 38, 341–349 (2009).
Tapping, C. R., Ling, L. & Razack, A. PleurX drain use in the management of malignant ascites: safety, complications, long-term patency and factors predictive of success. Br. J. Radiol 85, 623–628 (2012).
Kmietowicz, Z. Cancer patients should have access to device to treat fluid retention at home, says NICE. BMJ 344, e2272 (2012).
Puiffe, M.-L. et al. Characterization of ovarian cancer ascites on cell. Invasion, proliferation, spheroid formation, and gene expression in an in vitro model of epithelial ovarian cancer. Neoplasia 9, 820–829 (2007).
Lee, J. m. & Kohn, E. C. Proteomics as a guiding tool for more effective personalized therapy. Ann. Oncol. 21, 205–210 (2010).
Morozova, O. & Marra, M. A. Applications of next-generation sequencing technologies in functional genomics. Genomics 92, 255–264 (2008).
Hetland, T. E. et al. Class III β-tubulin expression in advanced-stage serous ovarian carcinoma effusions is associated with poor survival and primary chemoresistance. Hum. Pathol. 42, 1019–1026 (2011).
Gillet, J.-P. et al. Clinical relevance of multidrug resistance gene expression in ovarian serous carcinoma effusions. Mol. Pharm. 8, 2080–2088 (2011).
Shepherd, T. G., Thériault, B. L., Campbell, E. J. & Nachtigal, M. W. Primary culture of ovarian surface epithelial cells and ascites-derived ovarian cancer cells from patients. Nature Protoc. 1, 2643–2649 (2007).
Hu, L., McArthur, C. & Jaffe, R. B. Ovarian cancer stem-like side-population cells are tumourigenic and chemoresistant. Br. J. Cancer 102, 1276–1283 (2010).
Mukhopadhyay, A. et al. Development of a functional assay for homologous recombination status in primary cultures of epithelial ovarian tumor and correlation with sensitivity to poly(ADP-ribose) polymerase inhibitors. Clin. Cancer Res. 16, 2344–2351 (2010).
Fong, P. C. et al. Poly(ADP)-ribose polymerase inhibition: frequent durable responses in BRCA carrier ovarian cancer correlating with platinum-free interval. J. Clin. Oncol. 28, 2512–2519 (2010).
Elattar, A. et al. Androgen receptor expression is a biological marker for androgen sensitivity in high grade serous epithelial ovarian cancer. Gynecol. Oncol. 124, 142–147 (2012).
Carden, C. P. et al. The association of PI3 kinase signaling and chemoresistance in advanced ovarian cancer. Mol. Cancer Ther. 11, 1609–1617 (2012).
Rizzo, S. et al. Ovarian cancer stem cell-like side populations are enriched following chemotherapy and overexpress EZH2. Mol. Cancer Ther. 10, 325–335 (2011).
Moserle, L. et al. The side population of ovarian cancer cells is a primary target of IFN-α antitumor effects. Cancer Res. 68, 5658–5668 (2008).
Meirelles, K. et al. Human ovarian cancer stem/progenitor cells are stimulated by doxorubicin but inhibited by Mullerian inhibiting substance. Proc. Natl Acad. Sci. USA 109, 2358–2363 (2012).
Latifi, A. et al. Isolation and characterization of tumor cells from the ascites of ovarian cancer patients: molecular phenotype of chemoresistant ovarian tumors. PLoS ONE 766, e46858 (2012).
Davidson, B. Proteomic analysis of malignant ovarian cancer effusions as a tool for biologic and prognostic profiling. Clin. Cancer Res. 12, 791–799 (2006).
Yap, T. A., Carden, C. P. & Kaye, S. B. Beyond chemotherapy: targeted therapies in ovarian cancer. Nature Rev. Cancer 9, 167–181 (2009).
Lee, J.-M., Han, J. J., Altwerger, G. & Kohn, E. C. Proteomics and biomarkers in clinical trials for drug development. J. Proteomics 74, 2632–2641 (2011).
Runyon, B. A. et al. The serum-ascites albumin gradient is superior to the exudate-transudate concept in the differential diagnosis of ascites. Ann. Intern. Med. 117, 215–220 (1992).
Sheid, B. Angiogenic effects of macrophages isolated from ascitic fluid aspirated from women with advanced ovarian cancer. Cancer Lett. 62, 153–158 (1992).
Starling, E. H. On the absorption of fluids from the connective tissue spaces. J. Physiol. 19, 312–326 (1896).
Cattau, E. L. Jr, Benjamin, S. B., Knuff, T. E. & Castell, D. O. The accuracy of the physical examination in the diagnosis of suspected ascites. JAMA 247, 1164–1166 (1982).
Inadomi, J., Cello, J. P. & Koch, J. Ultrasonographic determination of ascitic volume. Hepatology 24, 549–551 (1996).
Akriviadis, E. A. Hemoperitoneum in patients with ascites. Am. J. Gastroenterol. 92, 567–575 (1997).
von Riedenauer, W. B., Janjua, S. A., Kwon, D. S., Zhang, Z. & Velanovich, V. Immunohistochemical identification of primary peritoneal serous cystadenocarcinoma mimicking advanced colorectal carcinoma: a case report. J. Med. Case Rep. 166, 150 (2007).
Acknowledgements
The Drug Development Unit, the Institute of Cancer Research and the Royal Marsden Hospital NHS Foundation Trust acknowledge support from Cancer Research UK, through core support and an Experimental Cancer Medicine Centre grant and from the National Institute of Health Research, as a Biomedical Research Centre. E.K. acknowledges support from the Wellcome Trust as a clinical research fellow.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- Omentum
-
A fold of peritoneum connecting or supporting abdominal structures, such as the stomach and liver.
- Paracentesis
-
The procedure to remove fluid that has accumulated in the abdominal cavity (peritoneal fluid) by inserting a wide-bore needle through the abdominal wall.
- Satiety
-
A condition of being full beyond capacity.
- Starling's hypothesis of capillary haemodynamics
-
The direction and rate of fluid transfer between blood plasma in the capillary and fluid in the tissue spaces depend on the hydrostatic pressure on each side of the capillary wall, on the osmotic pressure of protein in plasma and in tissue fluid, and on the properties of the capillary walls as a filtering membrane.
Rights and permissions
About this article
Cite this article
Kipps, E., Tan, D. & Kaye, S. Meeting the challenge of ascites in ovarian cancer: new avenues for therapy and research. Nat Rev Cancer 13, 273–282 (2013). https://doi.org/10.1038/nrc3432
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrc3432
This article is cited by
-
Causal association between 637 human metabolites and ovarian cancer: a mendelian randomization study
BMC Genomics (2024)
-
Tropomyosin1 isoforms underlie epithelial to mesenchymal plasticity, metastatic dissemination, and resistance to chemotherapy in high-grade serous ovarian cancer
Cell Death & Differentiation (2024)
-
Advances in the treatment of malignant ascites in China
Supportive Care in Cancer (2024)
-
Ovarian hyperstimulation syndrome and pregnancy luteoma mimicking malignant ascites: a rare case report
Journal of Ovarian Research (2023)
-
Tumor microenvironment in ovarian cancer peritoneal metastasis
Cancer Cell International (2023)