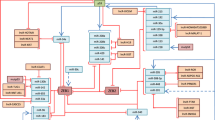
Key Points
-
p53 regulates the expression not only of protein-coding genes but also of non-coding RNAs, among which microRNAs (miRNAs) have been characterized as mediators of tumour suppression by p53 in the past 5 years. For example, the genes encoding the miR-34, miR-200, miR-15/16 and miR-192/194/215 families, as well as miR-145 and miR-107, are directly induced by p53.
-
p53 also regulates the processing of precursor miRNAs, either directly by binding to DROSHA or indirectly, as mutant p53 binds to and inactivates p63 and thereby downregulates the expression of DICER1. Furthermore, p53 may affect miRNA target gene selection by regulating mRNA-binding proteins, such as RNA-binding-motif protein 38 (RBM38).
-
p53-regulated miRNAs mediate tumour suppression and stress responses by regulating multiple key processes, such as cell cycle progression, migration, epithelial–mesenchymal transition, stemness, metabolism, differentiation and cell survival. miRNAs achieve this by directly targeting the translation and mRNA stability of central components of these processes.
-
In response to stress, such as oncogene activation and DNA damage, p53-regulated miRNAs are engaged in diverse types of feedforward and feedback loops that mediate amplification, robustness, fine-tuning and buffering of signals, and collectively contribute to appropriate cellular reactions. Accordingly, the expression and activity of p53 itself is also under the control of miRNAs.
-
Genes encoding p53-regulated miRNAs are often targets for inactivation by genetic and epigenetic mechanisms in human tumours, indicating that they are tumour suppressor genes.
-
Reintroduction of p53-regulated miRNAs into tumours with p53 mutation or miRNA inactivation may have therapeutic value, as this was shown to be effective in preclinical tumour models. Detection of the inactivation of p53-induced miRNAs in biopsy samples or body fluids may have diagnostic and/or prognostic value.
Abstract
In recent years, microRNAs (miRNAs) have been identified as mediators of tumour suppression and stress responses exerted by the p53 tumour suppressor. p53-regulated miRNAs contribute to tumour suppression by controlling the expression of central components of multiple processes, including cell cycle progression, epithelial–mesenchymal transition, stemness, metabolism, cell survival and angiogenesis. The expression and activity of p53 itself is also under the control of miRNAs. Finally, genetic and epigenetic alterations identified in the p53–miRNA network indicate that these pathways are important for the initiation and progression of tumours. In the future, knowledge about the p53–miRNA network may be able to be exploited for diagnostic and therapeutic approaches in cancer prevention and treatment.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Friedman, R. C., Farh, K. K., Burge, C. B. & Bartel, D. P. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 19, 92–105 (2009).
Lujambio, A. & Lowe, S. W. The microcosmos of cancer. Nature 482, 347–355 (2012).
Spizzo, R., Nicoloso, M. S., Croce, C. M. & Calin, G. A. SnapShot: microRNAs in cancer. Cell 137, 586–586.e1 (2009).
Lu, J. et al. MicroRNA expression profiles classify human cancers. Nature 435, 834–838 (2005).
Ravi, A. et al. Proliferation and tumorigenesis of a murine sarcoma cell line in the absence of DICER1. Cancer Cell 21, 848–855 (2012). An elegant study showing that miRNA function is not necessary for tumour formation in this mouse sarcoma tumour model.
Leung, A. K. & Sharp, P. A. microRNAs: a safeguard against turmoil? Cell 130, 581–585 (2007).
Leung, A. K. & Sharp, P. A. MicroRNA functions in stress responses. Mol. Cell 40, 205–215 (2010).
Soussi, T. TP53 mutations in human cancer: database reassessment and prospects for the next decade. Adv. Cancer Res. 110, 107–139 (2011).
Vogelstein, B., Lane, D. & Levine, A. J. Surfing the p53 network. Nature 408, 307–310 (2000).
Vaseva, Angelina, V. et al. p53 opens the mitochondrial permeability transition pore to trigger necrosis. Cell 149, 1536–1548 (2012).
Menendez, D., Inga, A. & Resnick, M. A. The expanding universe of p53 targets. Nature Rev. Cancer 9, 724–737 (2009).
Riley, T., Sontag, E., Chen, P. & Levine, A. Transcriptional control of human p53-regulated genes. Nature Rev. Mol. Cell Biol. 9, 402–412 (2008).
Rinn, J. L. & Huarte, M. To repress or not to repress: this is the guardian's question. Trends Cell Biol. 21, 344–353 (2011).
Kruse, J. P. & Gu, W. Modes of p53 regulation. Cell 137, 609–622 (2009).
Hermeking, H. The 14-3-3 cancer connection. Nature Rev. Cancer 3, 931–943 (2003).
Hermeking, H. p53 enters the microRNA world. Cancer Cell 12, 414–418 (2007).
Hermeking, H. The miR-34 family in cancer and apoptosis. Cell Death Differ. 17, 193–199 (2010).
Vousden, K. H. & Ryan, K. M. p53 and metabolism. Nature Rev. Cancer 9, 691–700 (2009).
Mendell, J. T. & Olson, E. N. MicroRNAs in stress signaling and human disease. Cell 148, 1172–1187 (2012).
Ebert, M. S. & Sharp, P. A. Roles for MicroRNAs in conferring robustness to biological processes. Cell 149, 515–524 (2012).
Suzuki, H. I. et al. Modulation of microRNA processing by p53. Nature 460, 529–533 (2009). This study shows the first evidence for a non-transcriptional role of p53 in miRNA processing.
Kumar, M. S. et al. Dicer1 functions as a haploinsufficient tumor suppressor. Genes Dev. 23, 2700–2704 (2009).
Mudhasani, R. et al. Loss of miRNA biogenesis induces p19Arf-p53 signaling and senescence in primary cells. J. Cell Biol. 181, 1055–1063 (2008). References 22 and 23 show that inhibition of DICER1 expression promotes metastasis, and that DICER1 loss elicits a p53-dependent arrest.
Su, X. et al. TAp63 suppresses metastasis through coordinate regulation of Dicer and miRNAs. Nature 467, 986–990 (2010). An important in vivo study showing that p63 suppresses metastasis by inducing DICER1 expression, which can be prevented by mutant p53 through binding to p63.
Leveille, N. et al. Selective inhibition of microRNA accessibility by RBM38 is required for p53 activity. Nature Commun. 2, 513 (2011). This paper reports the unexpected finding of another layer of complexity in the regulation of miRNA function by p53.
Tarasov, V. et al. Differential regulation of microRNAs by p53 revealed by massively parallel sequencing: miR-34a is a p53 target that induces apoptosis and G1-arrest. Cell Cycle 6, 1586–1593 (2007).
He, L. et al. A microRNA component of the p53 tumour suppressor network. Nature 447, 1130–1134 (2007).
Raver-Shapira, N. et al. Transcriptional activation of miR-34a contributes to p53-mediated apoptosis. Mol. Cell 26, 731–743 (2007).
Chang, T. C. et al. Transactivation of miR-34a by p53 broadly influences gene expression and promotes apoptosis. Mol. Cell 26, 745–752 (2007).
Bommer, G. T. et al. p53-mediated activation of miRNA34 candidate tumor-suppressor genes. Curr. Biol. 17, 1298–1307 (2007).
Corney, D. C., Flesken-Nikitin, A., Godwin, A. K., Wang, W. & Nikitin, A. Y. MicroRNA-34b and MicroRNA-34c are targets of p53 and cooperate in control of cell proliferation and adhesion-independent growth. Cancer Res. 67, 8433–8438 (2007). References 26 to 31 represent six almost simultaneous initial reports showing direct regulation of miR-34-encoding genes by p53.
Kaller, M. et al. Genome-wide characterization of miR-34a induced changes in protein and mRNA expression by a combined pulsed SILAC and micro-array analysis. Mol. Cell. Proteomics 10, M111.010462 (2011). This was the first genome-wide proteomic identification of miR-34 targets.
Lal, A. et al. Capture of microRNA-bound mRNAs identifies the tumor suppressor miR-34a as a regulator of growth factor signaling. PLoS Genet. 7, e1002363 (2011). This was the first genome-wide biochemical identification of miR-34 targets.
Sachdeva, M. et al. p53 represses c-Myc through induction of the tumor suppressor miR-145. Proc. Natl Acad. Sci. USA 106, 3207–3212 (2009).
Zhu, H. et al. EGFR signals downregulate tumor suppressors miR-143 and miR-145 in Western diet-promoted murine colon cancer: role of G1 regulators. Mol. Cancer Res. 9, 960–975 (2011).
Ragimov, N. et al. Wild-type but not mutant p53 can repress transcription initiation in vitro by interfering with the binding of basal transcription factors to the TATA motif. Oncogene 8, 1183–1193 (1993).
Hermeking, H. & Eick, D. Mediation of c-Myc-induced apoptosis by p53. Science 265, 2091–2093 (1994).
Lodygin, D. et al. Inactivation of miR-34a by aberrant CpG methylation in multiple types of cancer. Cell Cycle 7, 2591–2600 (2008). This was the first report showing frequent silencing of miR-34a by CpG methylation in primary tumours and diverse cancer cell lines.
Choi, Y. J. et al. miR-34 miRNAs provide a barrier for somatic cell reprogramming. Nature Cell Biol. 13, 1353–1360 (2011). This was the first evidence for the participation of miR-34 genes in the p53 barrier against iPSC formation.
Fabbri, M. et al. Association of a microRNA/TP53 feedback circuitry with pathogenesis and outcome of B-cell chronic lymphocytic leukemia. JAMA 305, 59–67 (2011).
Klein, U. et al. The DLEU2/miR-15a/16-1 cluster controls B cell proliferation and its deletion leads to chronic lymphocytic leukemia. Cancer Cell 17, 28–40 (2010). The first reported knockout study in mice revealing a tumour suppressor function for a p53-inducible miRNA-encoding gene.
Calin, G. A. et al. Frequent deletions and down-regulation of micro- RNA genes miR15 and miR16 at 13q14 in chronic lymphocytic leukemia. Proc. Natl Acad. Sci. USA 99, 15524–15529 (2002). This paper provides the initial identification of a genetic inactivation of an miRNA-encoding gene.
Bohlig, L. Friedrich, M. & Engeland, K. p53 activates the PANK1/miRNA-107 gene leading to downregulation of CDK6 and p130 cell cycle proteins. Nucleic Acids Res. 39, 440–453 (2011).
Staton, A. A., Knaut, H. & Giraldez, A. J. miRNA regulation of Sdf1 chemokine signaling provides genetic robustness to germ cell migration. Nature Genet. 43, 204–211 (2011).
Georges, S. A. et al. Coordinated regulation of cell cycle transcripts by p53-Inducible microRNAs, miR-192 and miR-215. Cancer Res. 68, 10105–10112 (2008).
Valastyan, S. & Weinberg, R. A. Tumor metastasis: molecular insights and evolving paradigms. Cell 147, 275–292 (2011).
Chang, C. J. et al. p53 regulates epithelial-mesenchymal transition and stem cell properties through modulating miRNAs. Nature Cell Biol. 13, 317–323 (2011).
Kim, T. et al. p53 regulates epithelial-mesenchymal transition through microRNAs targeting ZEB1 and ZEB2. J. Exp. Med. 208, 875–883 (2011). References 47 and 48 are the first demonstrations that p53 suppresses EMT and metastasis by inducing the miR-200 family genes.
Burk, U. et al. A reciprocal repression between ZEB1 and members of the miR-200 family promotes EMT and invasion in cancer cells. EMBO Rep. 9, 582–589 (2008).
Gregory, P. A. et al. The miR-200 family and miR-205 regulate epithelial to mesenchymal transition by targeting ZEB1 and SIP1. Nature Cell Biol. 10, 593–601 (2008).
Korpal, M., Lee, E. S., Hu, G. & Kang, Y. The miR-200 family inhibits epithelial-mesenchymal transition and cancer cell migration by direct targeting of E-cadherin transcriptional repressors ZEB1 and ZEB2. J. Biol. Chem. 283, 14910–14914 (2008).
Park, S. M., Gaur, A. B., Lengyel, E. & Peter, M. E. The miR-200 family determines the epithelial phenotype of cancer cells by targeting the E-cadherin repressors ZEB1 and ZEB2. Genes Dev. 22, 894–907 (2008).
Shimono, Y. et al. Downregulation of miRNA-200c links breast cancer stem cells with normal stem cells. Cell 138, 592–603 (2009).
Wellner, U. et al. The EMT-activator ZEB1 promotes tumorigenicity by repressing stemness-inhibiting microRNAs. Nature Cell Biol. 11, 1487–1495 (2009).
Neves, R. et al. Role of DNA methylation in miR-200c/141 cluster silencing in invasive breast cancer cells. BMC Res. Notes 3, 219 (2010).
Eades, G. et al. miR-200a regulates SIRT1 expression and epithelial to mesenchymal transition (EMT)-like transformation in mammary epithelial cells. J. Biol. Chem. 286, 25992–26002 (2011).
Siemens, H. et al. miR-34 and SNAIL form a double-negative feedback loop to regulate epithelial-mesenchymal transitions. Cell Cycle 10, 4256–4271 (2011). This was the first demonstration of a double-negative feedback loop between miR-34 and SNAIL in the regulation of EMT by p53.
Kim, N. H. et al. A p53/miRNA-34 axis regulates Snail1-dependent cancer cell epithelial-mesenchymal transition. J. Cell Biol. 195, 417–433 (2011). Together with reference 57 this study shows the regulation of the EMT-inducing factor SNAIL by miR-34.
Brabletz, T. MiR-34 and SNAIL: another double-negative feedback loop controlling cellular plasticity/EMT governed by p53. Cell Cycle 11, 215 (2012).
Dave, N. et al. Functional cooperation between Snail1 and twist in the regulation of ZEB1 expression during epithelial to mesenchymal transition. J. Biol. Chem. 286, 12024–12032 (2011).
Toyota, M. et al. Epigenetic silencing of microRNA-34b/c and B-cell translocation gene 4 is associated with CpG island methylation in colorectal cancer. Cancer Res. 68, 4123–4132 (2008).
Vogt, M. et al. Frequent concomitant inactivation of miR-34a and miR-34b/c by CpG methylation in colorectal, pancreatic, mammary, ovarian, urothelial, and renal cell carcinomas and soft tissue sarcomas. Virchows Arch. 458, 313–322 (2011).
Lujambio, A. et al. A microRNA DNA methylation signature for human cancer metastasis. Proc. Natl Acad. Sci. USA 105, 13556–13561 (2008).
Liu, C. et al. The microRNA miR-34a inhibits prostate cancer stem cells and metastasis by directly repressing CD44. Nature Med. 17, 211–215 (2011). This was the first systemic use of miR-34 mimetics to inhibit cancer stem cells and metastasis in mice.
Hwang, C. I. et al. Wild-type p53 controls cell motility and invasion by dual regulation of MET expression. Proc. Natl Acad. Sci. USA 108, 14240–14245 (2011). This paper provides a convincing in vivo idenification and analysis of a coherent forward feedback regulation of the MET gene by the combined activity of p53 and miR-34.
Mudduluru, G. et al. Regulation of Axl receptor tyrosine kinase expression by miR-34a and miR-199a/b in solid cancer. Oncogene 30, 2888–2899 (2011).
Martello, G. et al. A MicroRNA targeting dicer for metastasis control. Cell 141, 1195–1207 (2010).
Li, X. et al. MicroRNA-107, an oncogene microRNA that regulates tumour invasion and metastasis by targeting DICER1 in gastric cancer. J. Cell. Mol. Med. 15, 1887–1895 (2011).
Tapia, N. & Scholer, H. R. p53 connects tumorigenesis and reprogramming to pluripotency. J. Exp. Med. 207, 2045–2048 (2010).
Krizhanovsky, V. & Lowe, S. W. Stem cells: the promises and perils of p53. Nature 460, 1085–1086 (2009).
Jain, A. K. et al. p53 regulates cell cycle and microRNAs to promote differentiation of human embryonic stem cells. PLoS Biol. 10, e1001268 (2012).
Kim, N. H. et al. p53 and microRNA-34 are suppressors of canonical Wnt signaling. Sci. Signal. 4, ra71 (2011).
Thiery, J. P., Acloque, H., Huang, R. Y. & Nieto, M. A. Epithelial-mesenchymal transitions in development and disease. Cell 139, 871–890 (2009).
Ji, Q. et al. MicroRNA miR-34 inhibits human pancreatic cancer tumor-initiating cells. PLoS ONE 4, e6816 (2009).
Xu, N., Papagiannakopoulos, T., Pan, G., Thomson, J. A. & Kosik, K. S. MicroRNA-145 regulates OCT4, SOX2, and KLF4 and represses pluripotency in human embryonic stem cells. Cell 137, 647–658 (2009). This was the first demonstration of pluripotency inhibition by a p53-inducible miRNA in human embryonic stem cells.
Suh, S. O. et al. MicroRNA-145 is regulated by DNA methylation and p53 gene mutation in prostate cancer. Carcinogenesis 32, 772–778 (2011).
Gan, B. et al. FoxOs enforce a progression checkpoint to constrain mTORC1-activated renal tumorigenesis. Cancer Cell 18, 472–484 (2010).
Brabletz, S. et al. The ZEB1/miR-200 feedback loop controls Notch signalling in cancer cells. EMBO J. 30, 770–782 (2011).
Vallejo, D. M., Caparros, E. & Dominguez, M. Targeting Notch signalling by the conserved miR-8/200 microRNA family in development and cancer cells. EMBO J. 30, 756–769 (2011).
Yamakuchi, M., Ferlito, M. & Lowenstein, C. J. miR-34a repression of SIRT1 regulates apoptosis. Proc. Natl Acad. Sci. USA 105, 13421–13426 (2008).
Schickel, R., Park, S. M., Murmann, A. E. & Peter, M. E. miR-200c regulates induction of apoptosis through CD95 by targeting FAP-1. Mol. Cell 38, 908–915 (2010).
Cimmino, A. et al. miR-15 and miR-16 induce apoptosis by targeting BCL2. Proc. Natl Acad. Sci. USA 102, 13944–13949 (2005).
Pichiorri, F. et al. Downregulation of p53-inducible microRNAs 192, 194, and 215 impairs the p53/MDM2 autoregulatory loop in multiple myeloma development. Cancer Cell 18, 367–381 (2010).
Vega, S. et al. Snail blocks the cell cycle and confers resistance to cell death. Genes Dev. 18, 1131–1143 (2004).
Rottiers, V. & Näär, A. M. MicroRNAs in metabolism and metabolic disorders. Nature Rev. Mol. Cell Biol. 13, 239–250 (2012).
DeBerardinis, Ralph, J. & Thompson, Craig, B. Cellular metabolism and disease: what do metabolic outliers teach us? Cell 148, 1132–1144 (2012).
Koppenol, W. H., Bounds, P. L. & Dang, C. V. Otto Warburg's contributions to current concepts of cancer metabolism. Nature Rev. Cancer 11, 325–337 (2011).
Li, T. et al. Tumor suppression in the absence of p53-mediated cell-cycle arrest, apoptosis, and senescence. Cell 149, 1269–1283 (2012).
Li, W. Q. et al. The rno-miR-34 family is upregulated and targets ACSL1 in dimethylnitrosamine-induced hepatic fibrosis in rats. FEBS J. 278, 1522–1532 (2011).
Fantin, V. R., St-Pierre, J. & Leder, P. Attenuation of LDH-A expression uncovers a link between glycolysis, mitochondrial physiology, and tumor maintenance. Cancer Cell 9, 425–434 (2006).
Dang, C. V., Kim, J. W., Gao, P. & Yustein, J. The interplay between MYC and HIF in cancer. Nature Rev. Cancer 8, 51–56 (2008).
Menssen, A. et al. The c-MYC oncoprotein, the NAMPT enzyme, the SIRT1-inhibitor DBC1, and the SIRT1 deacetylase form a positive feedback loop. Proc. Natl Acad. Sci. USA 109, e187–e196 (2012).
Zhu, H. et al. The Lin28/let-7 axis regulates glucose metabolism. Cell 147, 81–94 (2011).
Yamakuchi, M. et al. P53-induced microRNA-107 inhibits HIF-1 and tumor angiogenesis. Proc. Natl Acad. Sci. USA 107, 6334–6339 (2010).
Roybal, J. D. et al. miR-200 Inhibits lung adenocarcinoma cell invasion and metastasis by targeting Flt1/VEGFR1. Mol. Cancer Res. 9, 25–35 (2011).
Panda, H. et al. Endometrial miR-200c is altered during transformation into cancerous states and targets the expression of ZEBs, VEGFA, FLT1, IKKβ, KLF9, and FBLN5. Reprod. Sci. 8 May 2012 (doi:10.1177/1933719112438448).
Yan, H. L. et al. Repression of the miR-17-92 cluster by p53 has an important function in hypoxia-induced apoptosis. EMBO J. 28, 2719–2732 (2009).
Le, M. T. N. et al. MicroRNA-125b is a novel negative regulator of p53. Genes Dev. 23, 862–876 (2009).
Le, M. T. et al. Conserved regulation of p53 network dosage by microRNA-125b occurs through evolving miRNA-target gene pairs. PLoS Genet. 7, e1002242 (2011).
Nishida, N. et al. MicroRNA miR-125b is a prognostic marker in human colorectal cancer. Int. J. Oncol. 38, 1437–1443 (2011).
Enomoto, Y. et al. Eμ/miR-125b transgenic mice develop lethal B-cell malignancies. Leukemia 25, 1849–1856 (2011).
Zhang, Y. et al. miR-125b is methylated and functions as a tumor suppressor by regulating the ETS1 proto-oncogene in human invasive breast cancer. Cancer Res. 71, 3552–3562 (2011).
Hu, W. et al. Negative regulation of tumor suppressor p53 by microRNA miR-504. Mol. Cell 38, 689–699 (2010).
Herrera-Merchan, A. et al. miR-33-mediated downregulation of p53 controls hematopoietic stem cell self-renewal. Cell Cycle 9, 3277–3285 (2010).
Tian, S. et al. MicroRNA-1285 inhibits the expression of p53 by directly targeting its 3′ untranslated region. Biochem. Biophys. Res. Commun. 396, 435–439 (2010).
Swarbrick, A. et al. miR-380-5p represses p53 to control cellular survival and is associated with poor outcome in MYCN-amplified neuroblastoma. Nature Med. 16, 1134–1140 (2010).
Kumar, M. et al. Negative regulation of the tumor suppressor p53 gene by microRNAs. Oncogene 30, 843–853 (2011).
Li, N. et al. A combined array-based comparative genomic hybridization and functional library screening approach identifies mir-30d as an oncomir in cancer. Cancer Res. 72, 154–164 (2012).
Chen, Q. R. et al. Systematic proteome analysis identifies transcription factor YY1 as a direct target of miR-34a. J. Proteome Res. 10, 479–487 (2011).
Lize, M., Klimke, A. & Dobbelstein, M. MicroRNA-449 in cell fate determination. Cell Cycle 10, 2874–2882 (2011).
Bou Kheir, T. et al. miR-449 inhibits cell proliferation and is down-regulated in gastric cancer. Mol. Cancer 10, 29 (2011).
Fornari, F. et al. MiR-122/cyclin G1 interaction modulates p53 activity and affects doxorubicin sensitivity of human hepatocarcinoma cells. Cancer Res. 69, 5761–5767 (2009).
Burns, D. M., D'Ambrogio, A., Nottrott, S. & Richter, J. D. CPEB and two poly(A) polymerases control miR-122 stability and p53 mRNA translation. Nature 473, 105–108 (2011). This paper provides an impressive demonstration of an alternative regulation of miR-122 by addition of adenine, which is important for p53 activation and senescence.
Braun, C. J. et al. p53-Responsive micrornas 192 and 215 are capable of inducing cell cycle arrest. Cancer Res. 68, 10094–10104 (2008).
Xiao, J., Lin, H., Luo, X. & Wang, Z. miR-605 joins p53 network to form a p53:miR-605:Mdm2 positive feedback loop in response to stress. EMBO J. 30, 524–532 (2011).
Park, S.-Y., Lee, J. H., Ha, M., Nam, J.-W. & Kim, V. N. miR-29 miRNAs activate p53 by targeting p85α and CDC42. Nature Struct. Mol. Biol. 16, 23–29 (2009).
Ugalde, A. P. et al. Aging and chronic DNA damage response activate a regulatory pathway involving miR-29 and p53. EMBO J. 30, 2219–2232 (2011).
Diederichs, S. & Haber, D. A. Sequence variations of microRNAs in human cancer: alterations in predicted secondary structure do not affect processing. Cancer Res. 66, 6097–6104 (2006).
Kuchenbauer, F. et al. In-depth characterization of the microRNA transcriptome in a leukemia progression model. Genome Res. 18, 1787–1797 (2008).
Mayr, C. & Bartel, D. P. Widespread shortening of 3'UTRs by alternative cleavage and polyadenylation activates oncogenes in cancer cells. Cell 138, 673–684 (2009).
Mayr, C., Hemann, M. T. & Bartel, D. P. Disrupting the pairing between let-7 and Hmga2 enhances oncogenic transformation. Science 315, 1576–1579 (2007).
Bui, T. V. & Mendell, J. T. Myc: maestro of microRNAs. Genes Cancer 1, 568–575 (2010).
Salmena, L., Poliseno, L., Tay, Y., Kats, L. & Pandolfi, P. P. A ceRNA hypothesis: the Rosetta Stone of a hidden RNA language? Cell 146, 353–358 (2011).
Tay, Y. et al. Coding-independent regulation of the tumor suppressor PTEN by competing endogenous mRNAs. Cell 147, 344–357 (2011).
Melo, S. A. et al. A genetic defect in exportin-5 traps precursor microRNAs in the nucleus of cancer cells. Cancer Cell 18, 303–315 (2010).
Melo, S. A. et al. A TARBP2 mutation in human cancer impairs microRNA processing and DICER1 function. Nature Genet. 41, 365–370 (2009).
Karube, Y. et al. Reduced expression of Dicer associated with poor prognosis in lung cancer patients. Cancer Sci. 96, 111–115 (2005).
Leaderer, D. et al. Genetic and epigenetic association studies suggest a role of microRNA biogenesis gene exportin-5 (XPO5) in breast tumorigenesis. Int. J. Mol. Epidemiol. Genet. 2, 9–18 (2011).
Merritt, W. M. et al. Dicer, Drosha, and outcomes in patients with ovarian cancer. N. Engl. J. Med. 359, 2641–2650 (2008).
Rio Frio, T. et al. DICER1 mutations in familial multinodular goiter with and without ovarian Sertoli-Leydig cell tumors. JAMA 305, 68–77 (2011).
Hill, D. A. et al. DICER1 mutations in familial pleuropulmonary blastoma. Science 325, 965 (2009).
Prosser, H. M., Koike-Yusa, H., Cooper, J. D., Law, F. C. & Bradley, A. A resource of vectors and ES cells for targeted deletion of microRNAs in mice. Nature Biotech. 29, 840–845 (2011).
Dow, L. E. et al. A pipeline for the generation of shRNA transgenic mice. Nature Protoc. 7, 374–393 (2012).
Baylin, S. B. & Jones, P. A. A decade of exploring the cancer epigenome — biological and translational implications. Nature Rev. Cancer 11, 726–734 (2011).
Kalimutho, M. et al. Epigenetically silenced miR-34b/c as a novel faecal-based screening marker for colorectal cancer. Br. J. Cancer 104, 1770–1778 (2011).
Kamimae, S. et al. Epigenetic alteration of DNA in mucosal wash fluid predicts invasiveness of colorectal tumors. Cancer Prev. Res. 4, 674–683 (2011). References 135 and 136 provide instructive examples of the feasibility of miRNA promoter methylation detection as a highly specific and sensitive tool for colorectal cancer diagnostics.
Feinberg, A. P., Ohlsson, R. & Henikoff, S. The epigenetic progenitor origin of human cancer. Nature Rev. Genet. 7, 21–33 (2006).
Fearon, E. R. & Vogelstein, B. A genetic model for colorectal tumorigenesis. Cell 61, 759–767 (1990).
Kasinski, A. L. & Slack, F. J. MicroRNAs en route to the clinic: progress in validating and targeting microRNAs for cancer therapy. Nature Rev. Cancer 11, 849–864 (2011).
Trang, P. et al. Systemic delivery of tumor suppressor microRNA mimics using a neutral lipid emulsion inhibits lung tumors in mice. Mol. Ther. 19, 1116–1122 (2011).
Tivnan, A. et al. Inhibition of neuroblastoma tumor growth by targeted delivery of microRNA-34a using anti-disialoganglioside GD2 coated nanoparticles. PLoS ONE 7, e38129 (2012). References 140 and 141 describe successful miRNA replacement therapies in preclinical mouse models of established lung tumours and neuroblastomas. Systemically administered miRNAs were well tolerated and effective.
Fabian, M. R., Sonenberg, N. & Filipowicz, W. Regulation of mRNA translation and stability by microRNAs. Annu. Rev. Biochem. 79, 351–379 (2010).
Bartel, D. P. MicroRNAs: target recognition and regulatory functions. Cell 136, 215–233 (2009).
Bandi, N. & Vassella, E. miR-34a and miR-15a/16 are co-regulated in non-small cell lung cancer and control cell cycle progression in a synergistic and Rb-dependent manner. Mol. Cancer 10, 55 (2011).
Chim, C. S. et al. Epigenetic inactivation of the miR-34a in hematological malignancies. Carcinogenesis 31, 745–750 (2010).
Corney, D. C. et al. Frequent downregulation of miR-34 family in human ovarian cancers. Clin. Cancer Res. 16, 1119–1128 (2010).
Kubo, T. et al. Epigenetic silencing of microRNA-34b/c plays an important role in the pathogenesis of malignant pleural mesothelioma. Clin. Cancer Res. 17, 4965–4974 (2011).
Chen, X. et al. CpG island methylation status of miRNAs in esophageal squamous cell carcinoma. Int. J. Cancer 130, 1607–1613 (2012).
Welch, C., Chen, Y. & Stallings, R. L. MicroRNA-34a functions as a potential tumor suppressor by inducing apoptosis in neuroblastoma cells. Oncogene 26, 5017–5022 (2007).
Gallardo, E. et al. miR-34a as a prognostic marker of relapse in surgically resected non-small-cell lung cancer. Carcinogenesis 30, 1903–1909 (2009).
Rucker, F. G. et al. Altered miRNA and gene expression in acute myeloid leukemia with complex karyotype identify networks of prognostic relevance. Leukemia 19 Jul 2012 (doi:10.1038/leu.2012.208).
Suzuki, H. et al. Methylation-associated silencing of microRNA-34b/c in gastric cancer and its involvement in an epigenetic field defect. Carcinogenesis 31, 2066–2073 (2010).
Wang, Z. et al. DNA hypermethylation of microRNA-34b/c has prognostic value for stage non-small cell lung cancer. Cancer Biol. Ther. 11, 490–496 (2011).
Cai, K. M. et al. Hsa-miR-34c suppresses growth and invasion of human laryngeal carcinoma cells via targeting c-Met. Int. J. Mol. Med. 25, 565–571 (2010).
Leucci, E. et al. MYC translocation-negative classical Burkitt lymphoma cases: an alternative pathogenetic mechanism involving miRNA deregulation. J. Pathol. 216, 440–450 (2008).
Dong, F. & Lou, D. MicroRNA-34b/c suppresses uveal melanoma cell proliferation and migration through multiple targets. Mol Vis 18, 537–546 (2012).
Calin, G. A. et al. A MicroRNA signature associated with prognosis and progression in chronic lymphocytic leukemia. N. Engl. J. Med. 353, 1793–1801 (2005).
Bonci, D. et al. The miR-15a-miR-16-1 cluster controls prostate cancer by targeting multiple oncogenic activities. Nature Med. 14, 1271–1277 (2008).
Bandi, N. et al. miR-15a and miR-16 are implicated in cell cycle regulation in a Rb-dependent manner and are frequently deleted or down-regulated in non-small cell lung cancer. Cancer Res. 69, 5553–5559 (2009).
Amaral, F. C. et al. MicroRNAs differentially expressed in ACTH-secreting pituitary tumors. J. Clin. Endocrinol. Metab. 94, 320–323 (2009).
Bhattacharya, R. et al. MiR-15a and MiR-16 control Bmi-1 expression in ovarian cancer. Cancer Res. 69, 9090–9095 (2009).
Bottoni, A. et al. miR-15a and miR-16-1 down-regulation in pituitary adenomas. J. Cell. Physiol. 204, 280–285 (2005).
Zhang, X. J. et al. Dysregulation of miR-15a and miR-214 in human pancreatic cancer. J. Hematol. Oncol. 3, 46 (2010).
Musumeci, M. et al. Control of tumor and microenvironment cross-talk by miR-15a and miR-16 in prostate cancer. Oncogene 30, 4231–4242 (2011).
Porkka, K. P. et al. The miR-15a-miR-16-1 locus is homozygously deleted in a subset of prostate cancers. Genes Chromosomes Cancer 50, 499–509 (2011).
Earle, J. S. et al. Association of microRNA expression with microsatellite instability status in colorectal adenocarcinoma. J. Mol. Diagn. 12, 433–440 (2010).
Wong, T. S. et al. Mature miR-184 as potential oncogenic microRNA of squamous cell carcinoma of tongue. Clin. Cancer Res. 14, 2588–2592 (2008).
Careccia, S. et al. A restricted signature of miRNAs distinguishes APL blasts from normal promyelocytes. Oncogene 28, 4034–4040 (2009).
Lee, K. H. et al. Epigenetic silencing of MicroRNA miR-107 regulates cyclin-dependent kinase 6 expression in pancreatic cancer. Pancreatology 9, 293–301 (2009).
Pallasch, C. P. et al. miRNA deregulation by epigenetic silencing disrupts suppression of the oncogene PLAG1 in chronic lymphocytic leukemia. Blood 114, 3255–3264 (2009).
Karaayvaz, M. et al. Prognostic significance of miR-215 in colon cancer. Clin. Colorectal Cancer 10, 340–347 (2011).
Kahlert, C. et al. Invasion front-specific expression and prognostic significance of microRNA in colorectal liver metastases. Cancer Sci. 102, 1799–1807 (2011).
Tellez, C. S. et al. EMT and stem cell-like properties associated with miR-205 and miR-200 epigenetic silencing are early manifestations during carcinogen-induced transformation of human lung epithelial cells. Cancer Res. 71, 3087–3097 (2011).
Ceppi, P. et al. Loss of miR-200c expression induces an aggressive, invasive, and chemoresistant phenotype in non-small cell lung cancer. Mol. Cancer Res. 8, 1207–1216 (2010).
Wiklund, E. D. et al. Coordinated epigenetic repression of the miR-200 family and miR-205 in invasive bladder cancer. Int. J. Cancer 128, 1327–1334 (2011).
Du, Y. et al. Down-regulation of miR-141 in gastric cancer and its involvement in cell growth. J. Gastroenterol. 44, 556–561 (2009).
Vrba, L. et al. Role for DNA methylation in the regulation of miR-200c and miR-141 expression in normal and cancer cells. PLoS ONE 5, e8697 (2010).
Hu, X. et al. A miR-200 microRNA cluster as prognostic marker in advanced ovarian cancer. Gynecol. Oncol. 114, 457–464 (2009).
Davalos, V. et al. Dynamic epigenetic regulation of the microRNA-200 family mediates epithelial and mesenchymal transitions in human tumorigenesis. Oncogene 31, 2062–2074 (2011).
Hur, K. et al. MicroRNA-200c modulates epithelial-to-mesenchymal transition (EMT) in human colorectal cancer metastasis. Gut 10 Jul 2012 (doi:10.1136/gutjnl-2011-301846).
Kim, M. S. et al. Somatic mutations and losses of expression of microRNA regulation-related genes AGO2 and TNRC6A in gastric and colorectal cancers. J. Pathol. 221, 139–146 (2010).
Bousquet, M. et al. Myeloid cell differentiation arrest by miR-125b-1 in myelodysplastic syndrome and acute myeloid leukemia with the t(2;11)(p21;q23) translocation. J. Exp. Med. 205, 2499–2506 (2008).
Acknowledgements
I apologize to authors whose contributions were not directly cited owing to space limitations. Work in the Hermeking laboratory is supported by the German-Israeli-Science-Foundation (GIF), the Rudolf-Bartling-Stiftung, the Deutsche Krebshilfe, the Deutsches Konsortium für translationale Krebsforschung (DKTK) and the Deutsche Forschungsgemeinschaft (DFG), Germany.
Author information
Authors and Affiliations
Ethics declarations
Competing interests
The author declares no competing financial interests.
Related links
Glossary
- Cluster
-
In the genome, microRNAs (miRNAs) are often organized in clusters, in which several different miRNAs are encoded by the same primary transcript miRNA. These often belong to one miRNA family.
- Coherent feedforward loops
-
In these regulatory circuits a transcription factor inhibits expression of a target gene and also activates a microRNA (miRNA) that is specific for the mRNA encoded by this gene. Even if the transcription factor is transiently ineffective, the mRNA will be repressed by the miRNA, conferring fidelity to the downregulation of the target gene.
- Incoherent feedforward loops
-
Regulatory circuits in which the microRNA and its target mRNA are transcriptionally co-induced, resulting in a more robust expression of the protein encoded by the mRNA.
- Haploinsufficient
-
A gene for which the loss of one allele in diploid organisms confers a detectable phenotype.
- Premature senescence
-
A permanent cell cycle arrest that, in a similar way to apoptosis, is thought to protect the organism from detrimental effects of transformed cells by precluding any propagation of damaged or mutant cells.
- TP63
-
The TP53, TP63 and TP73 genes constitute the p53 gene family, with highly related members displaying divergent regulation and function.
- Epithelial–mesenchymal transition
-
(EMT). The conversion of polarized, immotile epithelial cells to motile mesenchymal cells. It is characterized by the loss of adhesion, the repression of E-cadherin, the expression of mesenchymal markers and increased cell motility.
- CpG methylation
-
In tumours, this DNA modification occurs in CpG-rich regions that are often located proximally to the transcription start site of genes. In most mammalian genes, these CpG islands are normally maintained free of DNA methylation. In cancer cells, CpG islands of various tumour suppressor genes are frequently densely methylated, which results in repression of transcription.
- Pluripotency
-
A state of embryonic stem cells that can give rise to any fetal or adult cell type. Induced pluripotent stem cells are derived from non-pluripotent somatic cells by the simultaneous expression of a set of genes, typically the well-characterized pluripotency transcription factors OCT4, SOX2, KLF4 and MYC.
- Locked nucleic acid
-
(LNA). A synthetic olignucleotide in which the ribose sugar is modified with a methylene bridge between the 2′ oxygen and 4′ carbon atoms. This bridge 'locks' the sugar in the 3′-endo conformation seen in A-form DNA. LNAs display remarkably increased thermodynamic stability and enhanced nucleic acid recognition compared with physiological nucleic acids.
- Hypoxia-inducible factors
-
(HIFs). When HIF1 is stabilized by hypoxic conditions it upregulates several genes by binding to HIF-responsive elements (HREs) in their promoters to promote survival in low-oxygen conditions. The HIF targets include glycolysis enzymes and vascular endothelial growth factor (VEGF), which promotes angiogenesis.
- Seed region
-
Nucleotides 2–7 of the microRNA, typically with 100% complementarity to the target mRNA.
- Orthotopic model
-
A tissue or tumour transplant that is grafted into its normal anatomical site.
- miRNA sponges
-
Synthetic or naturally occurring microRNA (miRNA) target sequences that soak up miRNAs and thereby prevent them from inhibiting their functionally relevant target mRNAs.
- Microsatellite instability
-
Mutations in short motifs of tandemly repeated nucleotides that result from replication slippage and deficient mismatch repair.
- Antagomirs
-
Chemically engineered, single-stranded RNA oligonucleotides that are complementary to microRNAs (miRNAs), and that have modifications that prevent Argonaute 2 (AGO2)-mediated cleavage. They presumably inhibit miRNAs by irreversibly binding.
Rights and permissions
About this article
Cite this article
Hermeking, H. MicroRNAs in the p53 network: micromanagement of tumour suppression. Nat Rev Cancer 12, 613–626 (2012). https://doi.org/10.1038/nrc3318
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrc3318
This article is cited by
-
Blood-based microRNA profiling unveils complex molecular dynamics in breast cancer
Journal of Applied Genetics (2024)
-
Regulation and signaling pathways in cancer stem cells: implications for targeted therapy for cancer
Molecular Cancer (2023)
-
Curcumin activates a ROS/KEAP1/NRF2/miR-34a/b/c cascade to suppress colorectal cancer metastasis
Cell Death & Differentiation (2023)
-
Comprehensive circulating microRNA profile as a supersensitive biomarker for early-stage lung cancer screening
Journal of Cancer Research and Clinical Oncology (2023)
-
Circulating noncoding RNAs: promising biomarkers in liquid biopsy for the diagnosis, prognosis, and therapy of NSCLC
Discover Oncology (2023)