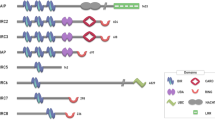
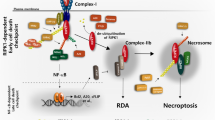
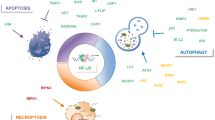
Key Points
-
Alterations in inhibitor of apoptosis (IAP) proteins are prevalent in many types of human cancer and are associated with chemoresistance, disease progression and poor prognosis.
-
IAPs are best known for their ability to regulate caspases; however, IAPs also influence a multitude of other cellular processes.
-
Possibly the most important contribution of IAPs to cell survival and tumorigenesis resides in the ability of cIAP1, cIAP2 and XIAP to regulate ubiquitin-dependent activation of nuclear factor-κB (NF-κB) and innate immune responses.
-
Constitutive activation of NF-κB and chronic inflammation both have a major role in tumour development and are seen in most tumour types, including leukaemia, lymphomas and solid tumours.
-
NF-κB can be activated through the canonical and non-canonical signal transduction cascade, and cIAPs are crucial regulators of both these pathways.
-
cIAPs are also indispensable in protecting cancer cells from the lethal effects of tumour necrosis factor receptor 1 activation.
-
Small-molecule IAP antagonists, termed Smac mimetics, cause the rapid depletion of cIAPs and show potent anti-tumorigenic activity in vitro and in vivo.
Abstract
The realization that alterations in inhibitor of apoptosis (IAP) proteins are found in many types of human cancer and are associated with chemoresistance, disease progression and poor prognosis, has sparked a worldwide frenzy in the development of small pharmacological inhibitors of IAPs. The development of such inhibitors has radically changed our knowledge of the signalling processes that are regulated by IAPs. Recent studies indicate that IAPs not only regulate caspases and apoptosis, but also modulate inflammatory signalling and immunity, mitogenic kinase signalling, proliferation and mitosis, as well as cell invasion and metastasis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Hanahan, D. & Weinberg, R. A. The hallmarks of cancer. Cell 100, 57–70 (2000).
LaCasse, E. C. et al. IAP-targeted therapies for cancer. Oncogene 27, 6252–6275 (2008).
Hunter, A. M., LaCasse, E. C. & Korneluk, R. G. The inhibitors of apoptosis (IAPs) as cancer targets. Apoptosis 12, 1543–1568 (2007).
Vince, J. E. et al. IAP antagonists target cIAP1 to induce TNFα-dependent apoptosis. Cell 131, 682–693 (2007).
Varfolomeev, E. et al. IAP antagonists induce autoubiquitination of c-IAPs, NF-κB activation, and TNFα-dependent apoptosis. Cell 131, 669–681 (2007).
Petersen, S. L. et al. Autocrine TNFα signaling renders human cancer cells susceptible to Smac-mimetic-induced apoptosis. Cancer Cell 12, 445–456 (2007). References 4–6 demonstrate that Smac mimetics trigger the rapid degradation of cIAPs and sensitize cancer cells to RIPK1-dependent TNFα-mediated apoptosis. Depletion of cIAPs is shown to activate non-canonical NF-κB signalling, which leads to TNFα production in some cell types.
Shaw, T. J., Lacasse, E. C., Durkin, J. P. & Vanderhyden, B. C. Downregulation of XIAP expression in ovarian cancer cells induces cell death in vitro and in vivo. Int. J. Cancer 122, 1430–1434 (2008).
McManus, D. C. et al. Loss of XIAP protein expression by RNAi and antisense approaches sensitizes cancer cells to functionally diverse chemotherapeutics. Oncogene 23, 8105–8117 (2004).
Hu, Y. et al. Antisense oligonucleotides targeting XIAP induce apoptosis and enhance chemotherapeutic activity against human lung cancer cells in vitro and in vivo. Clin. Cancer Res. 9, 2826–2836 (2003).
Keats, J. J. et al. Promiscuous mutations activate the noncanonical NF-κB pathway in multiple myeloma. Cancer Cell 12, 131–144 (2007).
Mehrotra, S. et al. IAP regulation of metastasis. Cancer Cell 17, 53–64 (2010). This paper shows that XIAP contributes to metastasis in vivo and cell invasion in vitro independently of caspase binding and inhibition. XIAP in complex with Survivin drives the activation of NF-κB to promote cell invasion and metastasis. cIAP1 and cIAP2 are also implicated in cancer cell invasion.
Dogan, T. et al. X-linked and cellular IAPs modulate the stability of C-RAF kinase and cell motility. Nature Cell Biol. 10, 1447–1455 (2008).
Srinivasula, S. M. & Ashwell, J. D. IAPs: what's in a name? Mol. Cell 30, 123–135 (2008).
Birnbaum, M. J., Clem, R. J. & Miller, L. K. An apoptosis-inhibiting gene from a nuclear polyhedrosis virus encoding a polypeptide with Cys/His sequence motifs. J. Virol. 68, 2521–2528 (1994).
Hinds, M. G., Norton, R. S., Vaux, D. L. & Day, C. L. Solution structure of a baculoviral inhibitor of apoptosis (IAP) repeat. Nature Struct. Biol. 6, 648–651 (1999).
Sun, C. et al. NMR structure and mutagenesis of the inhibitor-of-apoptosis protein XIAP. Nature 401, 818–822 (1999).
Yang, Y., Fang, S., Jensen, J. P., Weissman, A. M. & Ashwell, J. D. Ubiquitin protein ligase activity of IAPs and their degradation in proteasomes in response to apoptotic stimuli. Science 288, 874–877 (2000).
Gyrd-Hansen, M. et al. IAPs contain an evolutionarily conserved ubiquitin-binding domain that regulates NF-κB as well as cell survival and oncogenesis. Nature Cell Biol. 10, 1309–1317 (2008).
Blankenship, J. W. et al. Ubiquitin binding modulates IAP antagonist-stimulated proteasomal degradation of c-IAP1 and c-IAP2. Biochem. J. 417, 149–160 (2009). References 18 and 19 describe how IAPs harbour a UBA domain and interact preferentially with polyUb chains. Reference 18 describes how the UBA domain contributes to IAP-mediated regulation of NF-κB signalling, cell survival and oncogenesis. Reference 19 describes how the UBA domain is involved in proteasomal degradation of cIAPs in response to Smac mimetics.
Lin, S. C., Huang, Y., Lo, Y. C., Lu, M. & Wu, H. Crystal structure of the BIR1 domain of XIAP in two crystal forms. J. Mol. Biol. 372, 847–854 (2007).
Liu, Z. et al. Structural basis for binding of Smac/DIABLO to the XIAP BIR3 domain. Nature 408, 1004–1008 (2000).
Srinivasula, S. M. et al. A conserved XIAP-interaction motif in caspase-9 and Smac/DIABLO regulates caspase activity and apoptosis. Nature 410, 112–116 (2001).
Rothe, M., Pan, M. G., Henzel, W. J., Ayres, T. M. & Goeddel, D. V. The TNFR2-TRAF signaling complex contains two novel proteins related to baculoviral inhibitor of apoptosis proteins. Cell 83, 1243–1252 (1995). In this study, cIAP1 and cIAP2 were identified and cloned as components of the TNFR2 complex through their interaction with TRAF1 and TRAF2. More than a decade later, cIAPs are now recognized as essential for NF-κB activation and cell survival in response to TNFR activation.
Uren, A. G., Pakusch, M., Hawkins, C. J., Puls, K. L. & Vaux, D. L. Cloning and expression of apoptosis inhibitory protein homologs that function to inhibit apoptosis and/or bind tumor necrosis factor receptor-associated factors. Proc. Natl Acad. Sci. USA 93, 4974–4978 (1996).
Lu, M. et al. XIAP induces NF-κB activation via the BIR1/TAB1 interaction and BIR1 dimerization. Mol. Cell 26, 689–702 (2007).
Shi, Y. Mechanisms of caspase activation and inhibition during apoptosis. Mol. Cell 9, 459–470 (2002).
Pop, C. & Salvesen, G. S. Human caspases: activation, specificity, and regulation. J. Biol. Chem. 284, 21777–21781 (2009).
Eckelman, B. P., Salvesen, G. S. & Scott, F. L. Human inhibitor of apoptosis proteins: why XIAP is the black sheep of the family. EMBO Rep. 7, 988–994 (2006).
Deveraux, Q. L., Takahashi, R., Salvesen, G. S. & Reed, J. C. X-linked IAP is a direct inhibitor of cell-death proteases. Nature 388, 300–304 (1997).
Trapp, T. et al. Transgenic mice overexpressing XIAP in neurons show better outcome after transient cerebral ischemia. Mol. Cell. Neurosci. 23, 302–313 (2003).
Deveraux, Q. L. et al. IAPs block apoptotic events induced by caspase-8 and cytochrome c by direct inhibition of distinct caspases. EMBO J. 17, 2215–2223 (1998).
Conte, D., Liston, P., Wong, J. W., Wright, K. E. & Korneluk, R. G. Thymocyte-targeted overexpression of xiap transgene disrupts T lymphoid apoptosis and maturation. Proc. Natl Acad. Sci. USA 98, 5049–5054 (2001).
Takahashi, R. et al. A single BIR domain of XIAP sufficient for inhibiting caspases. J. Biol. Chem. 273, 7787–7790 (1998).
Wilkinson, J. C., Cepero, E., Boise, L. H. & Duckett, C. S. Upstream regulatory role for XIAP in receptor-mediated apoptosis. Mol. Cell. Biol. 24, 7003–7014 (2004).
Cummins, J. M. et al. X-linked inhibitor of apoptosis protein (XIAP) is a nonredundant modulator of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-mediated apoptosis in human cancer cells. Cancer Res. 64, 3006–3008 (2004).
Sasaki, H., Sheng, Y., Kotsuji, F. & Tsang, B. K. Down-regulation of X-linked inhibitor of apoptosis protein induces apoptosis in chemoresistant human ovarian cancer cells. Cancer Res. 60, 5659–5666 (2000).
Chawla-Sarkar, M. et al. Downregulation of Bcl-2, FLIP or IAPs (XIAP and survivin) by siRNAs sensitizes resistant melanoma cells to Apo2L/TRAIL-induced apoptosis. Cell Death Differ. 11, 915–923 (2004).
Hwang, C. et al. X-linked inhibitor of apoptosis deficiency in the TRAMP mouse prostate cancer model. Cell Death Differ. 15, 831–840 (2008).
Harlin, H., Reffey, S. B., Duckett, C. S., Lindsten, T. & Thompson, C. B. Characterization of XIAP-deficient mice. Mol. Cell. Biol. 21, 3604–3608 (2001).
Silke, J. et al. Direct inhibition of caspase 3 is dispensable for the anti-apoptotic activity of XIAP. EMBO J. 20, 3114–3123 (2001).
Huang, Y. et al. Structural basis of caspase inhibition by XIAP: differential roles of the linker versus the BIR domain. Cell 104, 781–790 (2001).
Suzuki, Y., Nakabayashi, Y. & Takahashi, R. Ubiquitin-protein ligase activity of X-linked inhibitor of apoptosis protein promotes proteasomal degradation of caspase-3 and enhances its anti-apoptotic effect in Fas-induced cell death. Proc. Natl Acad. Sci. USA 98, 8662–8667 (2001).
Chai, J. et al. Structural basis of caspase-7 inhibition by XIAP. Cell 104, 769–780 (2001).
Riedl, S. J. et al. Structural basis for the inhibition of caspase-3 by XIAP. Cell 104, 791–800 (2001).
Scott, F. L. et al. XIAP inhibits caspase-3 and -7 using two binding sites: evolutionarily conserved mechanism of IAPs. EMBO J. 24, 645–655 (2005).
Shiozaki, E. N. et al. Mechanism of XIAP-mediated inhibition of caspase-9. Mol.Cell 11, 519–527 (2003).
Deveraux, Q. L. et al. Cleavage of human inhibitor of apoptosis protein XIAP results in fragments with distinct specificities for caspases. EMBO J. 18, 5242–5251 (1999).
Schile, A. J., Garcia-Fernandez, M. & Steller, H. Regulation of apoptosis by XIAP ubiquitin-ligase activity. Genes Dev. 22, 2256–2266 (2008).
Jin, H. S. et al. cIAP1, cIAP2, and XIAP act cooperatively via nonredundant pathways to regulate genotoxic stress-induced nuclear factor-κB activation. Cancer Res. 69, 1782–1791 (2009).
Hawkins, C. J., Wang, S. L. & Hay, B. A. A cloning method to identify caspases and their regulators in yeast: identification of Drosophila IAP1 as an inhibitor of the Drosophila caspase DCP-1. Proc. Natl Acad. Sci. USA 96, 2885–2890 (1999).
Wang, S. L., Hawkins, C. J., Yoo, S. J., Muller, H. A. & Hay, B. A. The Drosophila caspase inhibitor DIAP1 is essential for cell survival and is negatively regulated by HID. Cell 98, 453–463 (1999).
Lisi, S., Mazzon, I. & White, K. Diverse domains of THREAD/DIAP1 are required to inhibit apoptosis induced by REAPER and HID in Drosophila. Genetics 154, 669–678 (2000).
Meier, P., Silke, J., Leevers, S. J. & Evan, G. I. The Drosophila caspase DRONC is regulated by DIAP1. EMBO J. 19, 598–611 (2000).
Hays, R., Wickline, L. & Cagan, R. Morgue mediates apoptosis in the Drosophila melanogaster retina by promoting degradation of DIAP1. Nature Cell Biol. 4, 425–431 (2002).
Muro, I., Hay, B. A. & Clem, R. J. The Drosophila DIAP1 protein is required to prevent accumulation of a continuously generated, processed form of the apical caspase DRONC. J. Biol. Chem. 277, 49644–49650 (2002).
Wilson, R. et al. The DIAP1 RING finger mediates ubiquitination of Dronc and is indispensable for regulating apoptosis. Nature Cell Biol. 4, 445–450 (2002).
Yoo, S. J. et al. Hid, Rpr and Grim negatively regulate DIAP1 levels through distinct mechanisms. Nature Cell Biol. 4, 416–424 (2002).
Chai, J. et al. Molecular mechanism of Reaper-Grim-Hid-mediated suppression of DIAP1-dependent Dronc ubiquitination. Nature Struct. Biol. 10, 892–898 (2003).
Ditzel, M. et al. Degradation of DIAP1 by the N-end rule pathway is essential for regulating apoptosis. Nature Cell Biol. 5, 467–473 (2003).
Yan, N., Wu, J. W., Chai, J., Li, W. & Shi, Y. Molecular mechanisms of DrICE inhibition by DIAP1 and removal of inhibition by Reaper, Hid and Grim. Nature Struct. Mol. Biol. 11, 420–428 (2004).
Yokokura, T. et al. Dissection of DIAP1 functional domains via a mutant replacement strategy. J. Biol. Chem. 279, 52603–52612 (2004).
Herman-Bachinsky, Y., Ryoo, H. D., Ciechanover, A. & Gonen, H. Regulation of the Drosophila ubiquitin ligase DIAP1 is mediated via several distinct ubiquitin system pathways. Cell Death Differ. 14, 861–871 (2007).
Ditzel, M. et al. Inactivation of effector caspases through nondegradative polyubiquitylation. Mol. Cell 32, 540–553 (2008).
Shapiro, P. J., Hsu, H. H., Jung, H., Robbins, E. S. & Ryoo, H. D. Regulation of the Drosophila apoptosome through feedback inhibition. Nature Cell Biol. 10, 1440–1446 (2008).
Ribaya, J. P. et al. The deubiquitinase emperor's thumb is a regulator of apoptosis in Drosophila. Dev. Biol. 329, 25–35 (2009).
Broemer, M. & Meier, P. Ubiquitin-mediated regulation of apoptosis. Trends Cell Biol. 19, 130–140 (2009).
Huang, H. et al. The inhibitor of apoptosis, cIAP2, functions as a ubiquitin-protein ligase and promotes in vitro monoubiquitination of caspases 3 and 7. J. Biol. Chem. 275, 26661–26664 (2000).
Choi, Y. E. et al. The E3 ubiquitin ligase cIAP1 binds and ubiquitinates caspases-3 and -7 via unique mechanisms at distinct steps in their processing. J. Biol. Chem. (2009).
Nathan, C. & Ding, A. Nonresolving inflammation. Cell 140, 871–882 (2010).
Grivennikov, S. I., Greten, F. R. & Karin, M. Immunity, inflammation, and cancer. Cell 140, 883–899 (2010).
Karin, M. & Greten, F. R. NF-κB: linking inflammation and immunity to cancer development and progression. Nature Rev. Immunol. 5, 749–759 (2005).
Perkins, N. D. Integrating cell-signalling pathways with NF-κB and IKK function. Nature Rev. Mol. Cell Biol. 8, 49–62 (2007).
Bonizzi, G. et al. Activation of IKKα target genes depends on recognition of specific kappaB binding sites by RelB:p52 dimers. EMBO J. 23, 4202–4210 (2004).
Bianchi, K. & Meier, P. A tangled web of ubiquitin chains: breaking news in TNF-R1 signaling. Mol. Cell 36, 736–742 (2009).
Bhoj, V. G. & Chen, Z. J. Ubiquitylation in innate and adaptive immunity. Nature 458, 430–437 (2009).
Leulier, F., Lhocine, N., Lemaitre, B. & Meier, P. The Drosophila inhibitor of apoptosis protein DIAP2 functions in innate immunity and is essential to resist gram-negative bacterial infection. Mol. Cell. Biol. 26, 7821–7831 (2006).
Gesellchen, V., Kuttenkeuler, D., Steckel, M., Pelte, N. & Boutros, M. An RNA interference screen identifies Inhibitor of Apoptosis Protein 2 as a regulator of innate immune signalling in Drosophila. EMBO Rep. 6, 979–984 (2005).
Kleino, A. et al. Inhibitor of apoptosis 2 and TAK1-binding protein are components of the Drosophila Imd pathway. EMBO J. 24, 3423–3434 (2005).
Paquette, N. et al. Caspase-mediated cleavage, IAP binding, and ubiquitination: linking three mechanisms crucial for Drosophila NF-κB signaling. Mol. Cell 37, 172–182 (2010).
Santoro, M. M., Samuel, T., Mitchell, T., Reed, J. C. & Stainier, D. Y. Birc2 (cIap1) regulates endothelial cell integrity and blood vessel homeostasis. Nature Genet. 39, 1397–1402 (2007).
Krieg, A. et al. XIAP mediates NOD signaling via interaction with RIP2. Proc. Natl Acad. Sci. USA 106, 14524–14529 (2009).
Bauler, L. D., Duckett, C. S. & O'Riordan, M. X. XIAP regulates cytosol-specific innate immunity to Listeria infection. PLoS Pathog. 4, e1000142 (2008).
Lewis, J. et al. Uncoupling of the signaling and caspase-inhibitory properties of X-linked inhibitor of apoptosis. J. Biol. Chem. 279, 9023–9029 (2004).
Ganesh, L. et al. The gene product Murr1 restricts HIV-1 replication in resting CD4+ lymphocytes. Nature 426, 853–857 (2003).
Burstein, E. et al. A novel role for XIAP in copper homeostasis through regulation of MURR1. EMBO J. 23, 244–254 (2004).
Birkey Reffey, S., Wurthner, J. U., Parks, W. T., Roberts, A. B. & Duckett, C. S. X-linked inhibitor of apoptosis protein functions as a cofactor in transforming growth factor-β signaling. J. Biol. Chem. 276, 26542–26549 (2001).
Yamaguchi, K. et al. XIAP, a cellular member of the inhibitor of apoptosis protein family, links the receptors to TAB1-TAK1 in the BMP signaling pathway. EMBO J. 18, 179–187 (1999).
Gaither, A. et al. A Smac mimetic rescue screen reveals roles for inhibitor of apoptosis proteins in tumor necrosis factor-α signaling. Cancer Res. 67, 11493–11498 (2007).
Bertrand, M. J. et al. cIAP1 and cIAP2 facilitate cancer cell survival by functioning as E3 ligases that promote RIP1 ubiquitination. Mol. Cell 30, 689–700 (2008). In this paper it is shown that cIAPs mediate TNFα-induced RIPK1 ubiquitylation that is important for the activation of the NF-κB kinase complex and NF-κB.
Mahoney, D. J. et al. Both cIAP1 and cIAP2 regulate TNFα-mediated NF-κB activation. Proc. Natl Acad. Sci. USA 105, 11778–11783 (2008).
Vallabhapurapu, S. et al. Nonredundant and complementary functions of TRAF2 and TRAF3 in a ubiquitination cascade that activates NIK-dependent alternative NF-κB signaling. Nature Immunol. 9, 1364–1370 (2008).
Varfolomeev, E. et al. c-IAP1 and c-IAP2 are critical mediators of tumor necrosis factor alpha (TNFalpha)-induced NF-kappaB activation. J. Biol. Chem. 283, 24295–24299 (2008).
Vince, J. E. et al. TWEAK-FN14 signaling induces lysosomal degradation of a cIAP1-TRAF2 complex to sensitize tumor cells to TNFα. J. Cell Biol. 182, 171–184 (2008).
Micheau, O. & Tschopp, J. Induction of TNF receptor I-mediated apoptosis via two sequential signaling complexes. Cell 114, 181–190 (2003).
Haas, T. L. et al. Recruitment of the linear ubiquitin chain assembly complex stabilizes the TNF-R1 signaling complex and is required for TNF-mediated gene induction. Mol. Cell 36, 831–844 (2009).
Kanayama, A. et al. TAB2 and TAB3 activate the NF-κB pathway through binding to polyubiquitin chains. Mol. Cell 15, 535–548 (2004).
Rahighi, S. et al. Specific recognition of linear ubiquitin chains by NEMO is important for NF-κB activation. Cell 136, 1098–1109 (2009).
Tokunaga, F. et al. Involvement of linear polyubiquitylation of NEMO in NF-κB activation. Nature Cell Biol. 11, 123–132 (2009).
Mace, P. D., Smits, C., Vaux, D. L., Silke, J. & Day, C. L. Asymmetric recruitment of cIAPs by TRAF2. J. Mol. Biol. 400, 8–15 (2010).
Zheng, C., Kabaleeswaran, V., Wang, Y., Cheng, G. & Wu, H. Crystal structures of the TRAF2: cIAP2 and the TRAF1: TRAF2: cIAP2 complexes: affinity, specificity, and regulation. Mol. Cell 38, 101–113 (2010).
Vince, J. E. et al. TRAF2 must bind to cellular inhibitors of apoptosis for tumor necrosis factor (tnf) to efficiently activate nf-{kappa}b and to prevent tnf-induced apoptosis. J. Biol. Chem. 284, 35906–35915 (2009).
Conze, D. B. et al. Posttranscriptional downregulation of c-IAP2 by the ubiquitin protein ligase c-IAP1 in vivo. Mol. Cell. Biol. 25, 3348–3356 (2005).
Conte, D. et al. Inhibitor of apoptosis protein cIAP2 is essential for lipopolysaccharide-induced macrophage survival. Mol. Cell. Biol. 26, 699–708 (2006).
Wang, L., Du, F. & Wang, X. TNF-α induces two distinct caspase-8 activation pathways. Cell 133, 693–703 (2008).
O'Donnell, M. A., Legarda-Addison, D., Skountzos, P., Yeh, W. C. & Ting, A. T. Ubiquitination of RIP1 regulates an NF-κB-independent cell-death switch in TNF signaling. Curr. Biol. 17, 418–424 (2007).
Wong, W. W. et al. RIPK1 is not essential for TNFR1-induced activation of NF-κB. Cell Death Differ. 17, 482–487 (2010).
Vercammen, D. et al. Inhibition of caspases increases the sensitivity of L929 cells to necrosis mediated by tumor necrosis factor. J. Exp. Med. 187, 1477–1485 (1998).
Vanden Berghe, T., Kalai, M., van Loo, G., Declercq, W. & Vandenabeele, P. Disruption of HSP90 function reverts tumor necrosis factor-induced necrosis to apoptosis. J. Biol. Chem. 278, 5622–5629 (2003).
Zhang, D. W. et al. RIP3, an energy metabolism regulator that switches TNF-induced cell death from apoptosis to necrosis. Science 325, 332–336 (2009).
He, S. et al. Receptor interacting protein kinase-3 determines cellular necrotic response to TNF-alpha. Cell 137, 1100–1111 (2009).
Zheng, L. et al. Competitive control of independent programs of tumor necrosis factor receptor-induced cell death by TRADD and RIP1. Mol. Cell. Biol. 26, 3505–3513 (2006).
Cho, Y. S. et al. Phosphorylation-driven assembly of the RIP1-RIP3 complex regulates programmed necrosis and virus-induced inflammation. Cell 137, 1112–1123 (2009).
Geserick, P. et al. Cellular IAPs inhibit a cryptic CD95-induced cell death by limiting RIP1 kinase recruitment. J. Cell Biol. 187, 1037–1054 (2009).
Bonizzi, G. & Karin, M. The two NF-κB activation pathways and their role in innate and adaptive immunity. Trends Immunol. 25, 280–288 (2004).
Hayden, M. S. & Ghosh, S. Shared principles in NF-κB signaling. Cell 132, 344–362 (2008).
Zarnegar, B. J. et al. Noncanonical NF-κB activation requires coordinated assembly of a regulatory complex of the adaptors cIAP1, cIAP2, TRAF2 and TRAF3 and the kinase NIK. Nature Immunol. 9, 1371–1378 (2008). This reference and reference 91 show that cIAP1 and cIAP2 form a complex with TRAF2 and TRAF3 and are responsible for the constitutive degradation of NIK to suppress non-canonical NF-κB activity. In response to ligation of CD40 or BAFF-R, cIAPs change substrate and degrade TRAF3, which results in the accumulation of NIK and NF-κB activation.
Winkles, J. A. The TWEAK-Fn14 cytokine-receptor axis: discovery, biology and therapeutic targeting. Nature Rev. Drug Discov. 7, 411–425 (2008).
Annunziata, C. M. et al. Frequent engagement of the classical and alternative NF-κB pathways by diverse genetic abnormalities in multiple myeloma. Cancer Cell 12, 115–130 (2007). This reference and reference 10 identify frequent and multiple genetic mutations that activate non-canonical NF-κB activity in multiple myeloma cells. Among these are recurrent biallelic deletions of BIRC2 and BIRC3 .
Yamaguchi, N. et al. Constitutive activation of nuclear factor-kappaB is preferentially involved in the proliferation of basal-like subtype breast cancer cell lines. Cancer Sci. 100, 1668–1674 (2009).
Wharry, C. E., Haines, K. M., Carroll, R. G. & May, M. J. Constitutive non-canonical NFκB signaling in pancreatic cancer cells. Cancer Biol. Ther. 8, 1567–1576 (2009).
Wu, G. et al. Structural basis of IAP recognition by Smac/DIABLO. Nature 408, 1008–1012 (2000).
Srinivasula, S. M. et al. Molecular determinants of the caspase-promoting activity of Smac/DIABLO and its role in the death receptor pathway. J. Biol. Chem. 275, 36152–36157 (2000).
Du, C., Fang, M., Li, Y., Li, L. & Wang, X. Smac, a mitochondrial protein that promotes cytochrome c-dependent caspase activation by eliminating IAP inhibition. Cell 102, 33–42 (2000).
Verhagen, A. M. et al. Identification of DIABLO, a mammalian protein that promotes apoptosis by binding to and antagonizing IAP proteins. Cell 102, 43–53 (2000).
Cheung, H. H., Mahoney, D. J., Lacasse, E. C. & Korneluk, R. G. Down-regulation of c-FLIP Enhances death of cancer cells by smac mimetic compound. Cancer Res. 69, 7729–7738 (2009).
Dineen, S. P. et al. Smac mimetic increases chemotherapy response and improves survival in mice with pancreatic cancer. Cancer Res. 70, 2852–2861 (2010).
Weisberg, E. et al. Potentiation of antileukemic therapies by Smac mimetic, LBW242: effects on mutant FLT3-expressing cells. Mol. Cancer Ther. 6, 1951–1961 (2007).
Lu, J. et al. SM-164: a novel, bivalent Smac mimetic that induces apoptosis and tumor regression by concurrent removal of the blockade of cIAP-1/2 and XIAP. Cancer Res. 68, 9384–9393 (2008).
Imoto, I. et al. Identification of cIAP1 as a candidate target gene within an amplicon at 11q22 in esophageal squamous cell carcinomas. Cancer Res. 61, 6629–6634 (2001).
Dai, Z. et al. A comprehensive search for DNA amplification in lung cancer identifies inhibitors of apoptosis cIAP1 and cIAP2 as candidate oncogenes. Hum. Mol. Genet. 12, 791–801 (2003).
Snijders, A. M. et al. Rare amplicons implicate frequent deregulation of cell fate specification pathways in oral squamous cell carcinoma. Oncogene 24, 4232–4242 (2005).
Reardon, D. A. et al. Extensive genomic abnormalities in childhood medulloblastoma by comparative genomic hybridization. Cancer Res. 57, 4042–4047 (1997).
Weber, R. G., Sommer, C., Albert, F. K., Kiessling, M. & Cremer, T. Clinically distinct subgroups of glioblastoma multiforme studied by comparative genomic hybridization. Lab. Invest. 74, 108–119 (1996).
Bashyam, M. D. et al. Array-based comparative genomic hybridization identifies localized DNA amplifications and homozygous deletions in pancreatic cancer. Neoplasia 7, 556–562 (2005).
Ma, O. et al. MMP13, Birc2 (cIAP1), and Birc3 (cIAP2), amplified on chromosome 9, collaborate with p53 deficiency in mouse osteosarcoma progression. Cancer Res. 69, 2559–2567 (2009).
Zender, L. et al. Identification and validation of oncogenes in liver cancer using an integrative oncogenomic approach. Cell 125, 1253–1267 (2006). In this paper, the amplicon encoding cIAP1, cIAP2 and YAP is found to be spontaneously amplified in this Myc-driven HCC model, and cIAP1 and YAP1 function as oncogenes. The amplicon identified is syntenic, with amplicons identified in human cancers including HCC.
Xu, L. et al. c-IAP1 cooperates with Myc by acting as a ubiquitin ligase for Mad1. Mol. Cell 28, 914–922 (2007).
Soucek, L. et al. Mast cells are required for angiogenesis and macroscopic expansion of Myc-induced pancreatic islet tumors. Nature Med. 13, 1211–1218 (2007).
Murdoch, C., Muthana, M., Coffelt, S. B. & Lewis, C. E. The role of myeloid cells in the promotion of tumour angiogenesis. Nature Rev. Cancer 8, 618–631 (2008).
Kim, S. et al. Carcinoma-produced factors activate myeloid cells through TLR2 to stimulate metastasis. Nature 457, 102–106 (2009).
Wu, Y. & Zhou, B. P. TNF-α/NF-κB/Snail pathway in cancer cell migration and invasion. Br. J. Cancer 102, 639–644 (2010).
Lin, W. W. & Karin, M. A cytokine-mediated link between innate immunity, inflammation, and cancer. J. Clin. Invest. 117, 1175–1183 (2007).
Mantovani, A., Allavena, P., Sica, A. & Balkwill, F. Cancer-related inflammation. Nature 454, 436–444 (2008).
Isaacson, P. G. & Du, M. Q. Gastrointestinal lymphoma: where morphology meets molecular biology. J. Pathol. 205, 255–274 (2005).
Zhou, H., Du, M. Q. & Dixit, V. M. Constitutive NF-kappaB activation by the t(11;18)(q21;q21) product in MALT lymphoma is linked to deregulated ubiquitin ligase activity. Cancer Cell 7, 425–431 (2005). This paper demonstrates that the MALT lymphoma associated t(11q21;18 q21) product cIAP2–MALT1 drives constitutive NF-κB activation through deregulated formation of K63-linked polyUb chains.
Wu, C. J. & Ashwell, J. D. NEMO recognition of ubiquitinated Bcl10 is required for T cell receptor-mediated NF-κB activation. Proc. Natl Acad. Sci. USA 105, 3023–3028 (2008).
Zhou, H. et al. Bcl10 activates the NF-κB pathway through ubiquitination of NEMO. Nature 427, 167–171 (2004).
Sun, L., Deng, L., Ea, C. K., Xia, Z. P. & Chen, Z. J. The TRAF6 ubiquitin ligase and TAK1 kinase mediate IKK activation by BCL10 and MALT1 in T lymphocytes. Mol. Cell 14, 289–301 (2004).
Oeckinghaus, A. et al. Malt1 ubiquitination triggers NF-κB signaling upon T-cell activation. EMBO J. 26, 4634–4645 (2007).
Lucas, P. C. et al. A dual role for the API2 moiety in API2-MALT1-dependent NF-κB activation: heterotypic oligomerization and TRAF2 recruitment. Oncogene 26, 5643–5654 (2007).
Takeuchi, O. & Akira, S. Pattern recognition receptors and inflammation. Cell 140, 805–820 (2010).
Tseng, P. H. et al. Different modes of ubiquitination of the adaptor TRAF3 selectively activate the expression of type I interferons and proinflammatory cytokines. Nature Immunol. 11, 70–75 (2010). This report demonstrates that cIAPs control the production of pro-inflammatory and pro-tumorigenic cytokines and chemokines in response to TLR4 activation, whereas cIAPs do not affect the production of anti-viral and anti-tumorigenic type I interferons.
Luo, J. L., Maeda, S., Hsu, L. C., Yagita, H. & Karin, M. Inhibition of NF-κB in cancer cells converts inflammation- induced tumor growth mediated by TNFα to TRAIL-mediated tumor regression. Cancer Cell 6, 297–305 (2004).
Podar, K., Chauhan, D. & Anderson, K. C. Bone marrow microenvironment and the identification of new targets for myeloma therapy. Leukemia 23, 10–24 (2009).
Chauhan, D. et al. Functional interaction of plasmacytoid dendritic cells with multiple myeloma cells: a therapeutic target. Cancer Cell 16, 309–323 (2009).
Rigaud, S. et al. XIAP deficiency in humans causes an X-linked lymphoproliferative syndrome. Nature 444, 110–114 (2006).
Sweeney, M. C., Wang, X., Park, J., Liu, Y. & Pei, D. Determination of the sequence specificity of XIAP BIR domains by screening a combinatorial peptide library. Biochemistry 45, 14740–14748 (2006).
Huang, Y., Rich, R. L., Myszka, D. G. & Wu, H. Requirement of both the second and third BIR domains for the relief of X-linked inhibitor of apoptosis protein (XIAP)-mediated caspase inhibition by Smac. J. Biol. Chem. 278, 49517–49522 (2003).
Boatright, K. M. et al. A unified model for apical caspase activation. Mol. Cell 11, 529–541 (2003).
Meier, P. & Vousden, K. H. Lucifer's labyrinth--ten years of path finding in cell death. Mol. Cell 28, 746–754 (2007).
White, K. et al. Genetic control of programmed cell death in Drosophila. Science 264, 677–683 (1994).
Bergmann, A., Agapite, J., McCall, K. & Steller, H. The Drosophila gene hid is a direct molecular target of Ras-dependent survival signaling. Cell 95, 331–341 (1998).
Kurada, P. & White, K. Ras promotes cell survival in Drosophila by downregulating hid expression. Cell 95, 319–329 (1998).
Brennecke, J., Hipfner, D. R., Stark, A., Russell, R. B. & Cohen, S. M. bantam encodes a developmentally regulated microRNA that controls cell proliferation and regulates the proapoptotic gene hid in Drosophila. Cell 113, 25–36 (2003).
Hegde, R. et al. The polypeptide chain-releasing factor GSPT1/eRF3 is proteolytically processed into an IAP-binding protein. J. Biol. Chem. 278, 38699–38706 (2003).
Suzuki, Y. et al. A serine protease, HtrA2, is released from the mitochondria and interacts with XIAP, inducing cell death. Mol. Cell 8, 613–621 (2001).
Holley, C. L., Olson, M. R., Colon-Ramos, D. A. & Kornbluth, S. Reaper eliminates IAP proteins through stimulated IAP degradation and generalized translational inhibition. Nature Cell Biol. 4, 439–444 (2002).
Crook, N. E., Clem, R. J. & Miller, L. K. An apoptosis-inhibiting baculovirus gene with a zinc finger-like motif. J. Virol. 67, 2168–2174 (1993).
Mace, P. D. et al. Structures of the cIAP2 RING domain reveal conformational changes associated with ubiquitin-conjugating enzyme (E2) recruitment. J. Biol. Chem. 283, 31633–31640 (2008).
Verhagen, A. M. et al. Identification of mammalian mitochondrial proteins that interact with IAPs via N-terminal IAP binding motifs. Cell Death Differ. 14, 348–357 (2007).
Acknowledgements
The authors would like to apologize to those whose work could not be cited or were cited only indirectly owing to space limitations. The authors would like to thank T. Nyman (BIR3-AVPI), M. Zvelebil (UBA) and C. Day (RING) for help with the structures, and members of the Meier Laboratory for helpful discussion and critical reading of the manuscript.
Author information
Authors and Affiliations
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Glossary
- Intrinsic apoptosis pathway
-
This pathway is dependent on mitochondria and is activated by developmental cues and cellular stresses such as DNA damage and oncogene activation. Pro-apoptotic BCL-2 family proteins facilitate the release of cytochrome c and other apoptogenic factors from the mitochondrial intermembrane space.
- Extrinsic apoptosis pathway
-
This pathway is initiated on ligation of cell surface receptors of the TNFR superfamily. Activation of these receptors triggers the assembly of death-inducing signalling complexes that serve as a platform to activate caspase 8 and caspase 10.
- Neo-amino-terminus
-
On proteolytic cleavage of a polypeptide, the amino acid immediately C terminal to the cleavage site becomes the new N terminal amino acid of the C terminal fragment.
- Canonical pathway
-
Signals that predominantly activate the RELA–p50 heterodimers.
- Non-canonical pathway
-
Signals that result in the activation of RELB–p52 heterodimers.
Rights and permissions
About this article
Cite this article
Gyrd-Hansen, M., Meier, P. IAPs: from caspase inhibitors to modulators of NF-κB, inflammation and cancer. Nat Rev Cancer 10, 561–574 (2010). https://doi.org/10.1038/nrc2889
Issue Date:
DOI: https://doi.org/10.1038/nrc2889
This article is cited by
-
LUBAC is required for RIG-I sensing of RNA viruses
Cell Death & Differentiation (2024)
-
Effect of regulatory cell death on the occurrence and development of head and neck squamous cell carcinoma
Biomarker Research (2023)
-
Drosophila caspases as guardians of host-microbe interactions
Cell Death & Differentiation (2023)
-
Molecular basis for nuclear accumulation and targeting of the inhibitor of apoptosis BIRC2
Nature Structural & Molecular Biology (2023)
-
Harnessing TRAIL-induced cell death for cancer therapy: a long walk with thrilling discoveries
Cell Death & Differentiation (2023)