Key Points
-
The close association between cell lineage and cancer phenotype has long been recognized. This link raises the possibility that cellular mechanisms that govern lineage proliferation and survival during development might also underlie tumorigenic mechanisms.
-
Many somatic genetic alterations show lineage-restricted patterns across human tumours, which indicates that genetic changes in cancer might be conditioned by the lineage programmes that are embedded in tumour precursor cells.
-
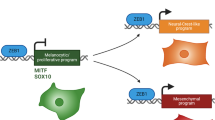
A convergence of lineage-based and genetic observations gives rise to a lineage-dependency (or lineage-addiction) model of human cancer, wherein tumour cells depend crucially on survival mechanisms that are programmed into lineage precursor cells during development, which might be affected by acquired genetic alterations. Unlike oncogene addiction, which invokes a dependency on a tumour-specific gain-of-function event, lineage addiction involves the persistence and/or deregulation of crucial lineage-survival mechanisms during carcinogenesis or tumour progression.
-
Presumably, lineage-dependency mechanisms that promote tumour progression involve master regulatory genes that also exert key developmental survival roles. Such genes can be termed lineage-survival oncogenes.
-
MITF (microphthalmia-associated transcription factor) and the androgen receptor are prototype lineage-survival oncogenes in melanoma and prostate cancer, respectively. A review of the scientific literature readily identifies several more genes with presumptive or predicted lineage-survival functions in different cancers.
-
Recognition of the lineage-dependency model might expand existing paradigms for tumour biology by emphasizing the importance of lineage in shaping key oncogenic mechanisms, thereby offering an explanatory framework for the distribution of genetic alterations in cancer. Targeting lineage dependencies as well as classical gain-of-function events might require combinatorial or synthetic-lethal therapeutic approaches to cancer.
Abstract
Although cell-lineage and differentiation models dominate tumour classification and treatment, the recognition that cancer is also a genomic disease has prompted a reconfiguration of cancer taxonomies according to molecular criteria. Recent evidence indicates that a synthesis of lineage-based and genetic paradigms might offer new insights into crucial and therapeutically pliable tumour dependencies. For example, MITF (microphthalmia-associated transcription factor), which is a master regulator of the melanocyte lineage, might become a melanoma oncogene when deregulated in certain genetic contexts. MITF and other lineage-survival genes therefore implicate lineage dependency (or lineage addiction) as a newly recognized mechanism that is affected by tumour genetic alterations.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Strausberg, R. L., Simpson, A. J., Old, L. J. & Riggins, G. J. Oncogenomics and the development of new cancer therapies. Nature 429, 469–474 (2004).
Fenaux, P. & Degos, L. Differentiation therapy for acute promyelocytic leukemia. N. Engl. J. Med. 337, 1076–1077 (1997).
Tallman, M. S. et al. All-trans-retinoic acid in acute promyelocytic leukemia N. Engl. J. Med. 337, 1021–1028 (1997).
Berman, J. J. Tumor taxonomy for the developmental lineage classification of neoplasms. BMC Cancer 4, 88 (2004).
Berman, J. Modern classification of neoplasms: reconciling differences between morphologic and molecular approaches. BMC Cancer 5, 100 (2005).
Thiery, J. P. Epithelial–mesenchymal transitions in tumour progression. Nature Rev. Cancer 2, 442–454 (2002).
Kang, Y. & Massague, J. Epithelial–mesenchymal transitions: twist in development and metastasis. Cell 118, 277–279 (2004).
Johnston, R. N., Pai, S. B. & Pai, R. B. The origin of the cancer cell: oncogeny reverses phylogeny. Biochem. Cell. Biol. 70, 831–834 (1992).
Gupta, P. B. et al. The melanocyte differentiation program predisposes to metastasis after neoplastic transformation. Nature Genet. 37, 1047–1054 (2005). This paper provides direct experimental evidence that lineage programming contributes to the metastatic phenotype in melanoma cells.
Muller, C. & Leutz, A. Chromatin remodeling in development and differentiation. Curr. Opin. Genet. Dev. 11, 167–174 (2001).
Kluger, Y., Lian, Z., Zhang, X., Newburger, P. E. & Weissman, S. M. A panorama of lineage-specific transcription in hematopoiesis. Bioessays 26, 1276–1287 (2004).
Nagamura-Inoue, T., Tamura, T. & Ozato, K. Transcription factors that regulate growth and differentiation of myeloid cells. Int. Rev. Immunol. 20, 83–105 (2001).
Warburton, D. et al. The molecular basis of lung morphogenesis. Mech. Dev. 92, 55–81 (2000).
Fraga, M. F. et al. Loss of acetylation at Lys16 and trimethylation at Lys20 of histone H4 is a common hallmark of human cancer. Nature Genet. 37, 391–400 (2005).
Strahl, B. D. & Allis, C. D. The language of covalent histone modifications. Nature 403, 41–45 (2000).
Wolffe, A. P. & Hayes, J. J. Chromatin disruption and modification. Nucleic Acids Res. 27, 711–720 (1999).
Bird, A. DNA methylation patterns and epigenetic memory. Genes Dev. 16, 6–21 (2002).
Vignali, M., Hassan, A. H., Neely, K. E. & Workman, J. L. ATP-dependent chromatin-remodeling complexes. Mol. Cell. Biol. 20, 1899–1910 (2000).
Ringrose, L. & Paro, R. Epigenetic regulation of cellular memory by the Polycomb and Trithorax group proteins. Annu. Rev. Genet. 38, 413–443 (2004).
Wolpert, L. Positional information and pattern formation in development. Dev. Genet. 15, 485–490 (1994).
Davis, P. K. & Brackmann, R. K. Chromatin remodeling and cancer. Cancer Biol. Ther. 2, 22–29 (2003).
Versteege, I. et al. Truncating mutations of hSNF5/INI1 in aggressive paediatric cancer. Nature 394, 203–206 (1998).
Sevenet, N. et al. Spectrum of hSNF5/INI1 somatic mutations in human cancer and genotype-phenotype correlations. Hum. Mol. Genet. 8, 2359–2368 (1999).
Grand, F. et al. Frequent deletion of hSNF5/INI1, a component of the SWI/SNF complex, in chronic myeloid leukemia. Cancer Res. 59, 3870–3874 (1999).
Sawa, M. et al. BMI-1 is highly expressed in M0-subtype acute myeloid leukemia. Int. J. Hematol. 82, 42–47 (2005).
Dukers, D. F. et al. Unique polycomb gene expression pattern in Hodgkin's lymphoma and Hodgkin's lymphoma-derived cell lines. Am. J. Pathol. 164, 873–881 (2004).
Abate-Shen, C. Deregulated homeobox gene expression in cancer: cause or consequence? Nature Rev. Cancer 2, 777–785 (2002).
Grier, D. G. et al. The pathophysiology of HOX genes and their role in cancer. J. Pathol. 205, 154–171 (2005).
Kho, A. T. et al. Conserved mechanisms across development and tumorigenesis revealed by a mouse development perspective of human cancers. Genes Dev. 18, 629–640 (2004). This paper shows that certain tumour subsets may be characterized by their molecular similarity to a particular developmental stage.
Hahn, W. C. & Weinberg, R. A. Rules for making human tumor cells. N. Engl. J. Med. 347, 1593–1603 (2002).
Weber, B. L. Cancer genomics. Cancer Cell 1, 37–47 (2002).
Davies, H. et al. Mutations of the BRAF gene in human cancer. Nature 417, 949–954 (2002). This paper represents one of the first large-scale sequencing efforts to identify a novel oncogene mutation.
Sieber, O. M., Tomlinson, S. R. & Tomlinson, I. P. Tissue, cell and stage specificity of (epi)mutations in cancers. Nature Rev. Cancer 5, 649–655 (2005).
Sharpless, E. & Chin, L. The INK4a/ARF locus and melanoma. Oncogene 22, 3092–3098 (2003).
Sharpless, N. E., Kannan, K., Xu, J., Bosenberg, M. W. & Chin, L. Both products of the mouse Ink4a/Arf locus suppress melanoma formation in vivo. Oncogene 22, 5055–5059 (2003).
Keyomarsi, K. & Pardee, A. B. Redundant cyclin overexpression and gene amplification in breast cancer cells. Proc. Natl Acad. Sci. USA 90, 1112–1116 (1993).
Bringuier, P. P., Tamimi, Y., Schuuring, E. & Schalken, J. Expression of cyclin D1 and EMS1 in bladder tumours; relationship with chromosome 11q13 amplification. Oncogene 12, 1747–1753 (1996).
Slamon, D. J. et al. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 235, 177–182 (1987).
Wong, A. J. et al. Increased expression of the epidermal growth factor receptor gene in malignant gliomas is invariably associated with gene amplification. Proc. Natl Acad. Sci. USA 84, 6899–6903 (1987).
Reissmann, P. T., Koga, H., Figlin, R. A., Holmes, E. C. & Slamon, D. J. Amplification and overexpression of the cyclin D1 and epidermal growth factor receptor genes in non-small-cell lung cancer. J. Cancer Res. Clin. Oncol. 125, 61–70 (1999).
Garraway, L. A. et al. Integrative genomic analyses identify MITF as a lineage survival oncogene amplified in malignant melanoma. Nature 436, 117–122 (2005). This paper demonstrated the power of combining genomic data sets for lineage-associated cancer gene discovery, and characterized the first lineage-survival oncogene.
Zhao, X. et al. Homozygous deletions and chromosome amplifications in human lung carcinomas revealed by single nucleotide polymorphism array analysis. Cancer Res. 65, 5561–5570 (2005).
Garraway, L. A. et al. 'Lineage addiction' in human cancer: lessons from integrated genomics. Cold Spring Harb. Symp. Quant. Biol. 70, 1–10 (2005).
Weinstein, I. B. Addiction to oncogenes — the Achilles heal of cancer. Science 297, 63–64 (2002).
Kantarjian, H. et al. Hematologic and cytogenetic responses to imatinib mesylate in chronic myelogenous leukemia. N. Engl. J. Med. 346, 645–652 (2002).
Demetri, G. D. et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N. Engl. J. Med. 347, 472–480 (2002).
Paez, J. G. et al. EGFR mutations in lung cancer: correlation with clinical response to gefinitib therapy. Science 304, 1497–1500 (2004).
Lynch, T. J. et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N. Engl. J. Med. 350, 2129–2139 (2004).
Morales, A. V., Barbas, J. A. & Nieto, M. A. How to become neural crest: from segregation to delamination. Semin. Cell Dev. Biol. 16, 655–662 (2005).
Dupin, E. & Le Douarin, N. M. Development of melanocyte precursors from the vertebrate neural crest. Oncogene 22, 3016–3023 (2003).
Widlund, H. R. & Fisher, D. E. Microphthalamia-associated transcription factor: a critical regulator of pigment cell development and survival. Oncogene 22, 3035–3041 (2003).
Goding, C. R. Mitf from neural crest to melanoma: signal transduction and transcription in the melanocyte lineage. Genes Dev. 14, 1712–1728 (2000).
Garraway, L. A. & Sellers, W. R. From integrated genomics to tumor lineage dependency. Cancer Res. 66, 2506–2508 (2006).
Du, J. et al. Critical role of CDK2 for melanoma growth linked to its melanocyte-specific transcriptional regulation by MITF. Cancer Cell 6, 565–576 (2004).
McGill, G. G. et al. Bcl2 regulation by the melanocyte master regulator Mitf modulates lineage survival and melanoma cell viability. Cell 109, 707–718 (2002). This paper was the first to suggest a mechanism by which MITF, the master melanocyte transcription factor, might exert a lineage-survival role.
Loercher, A. E., Tank, E. M., Delston, R. B. & Harbour, J. W. MITF links differentiation with cell cycle arrest in melanocytes by transcriptional activation of INK4A. J. Cell Biol. 168, 35–40 (2005).
Carreira, S. et al. Mitf cooperates with Rb1 and activates p21Cip1 expression to regulate cell cycle progression. Nature 433, 764–769 (2005). References 56 and 57 showed that MITF induces growth arrest in non-transformed cells, and that this arrest was dependent on the integrity of key cell-cycle inhibitory pathways.
Chin, L. The genetics of malignant melanoma: lessons from mouse and man. Nature Rev. Cancer 3, 559–570 (2003).
Hemesath, T. J., Price, E. R., Takemoto, C., Badalian, T. & Fisher, D. E. MAP kinase links the transcription factor Microphthalmia to c-Kit signalling in melanocytes. Nature 391, 298–301 (1998).
Omholt, K., Platz, A., Kanter, L., Ringborg, U. & Hansson, J. NRAS and BRAF mutations arise early during melanoma pathogenesis and are preserved throughout tumor progression. Clin Cancer Res 9, 6483–6488 (2003).
Reifenberger, J. et al. Frequent alterations of Ras signaling pathway genes in sporadic malignant melanomas. Int. J. Cancer 109, 377–384 (2004).
Heinlein, C. A. & Chang, C. Androgen receptor in prostate cancer. Endocr. Rev. 25, 276–308 (2004).
Waltregny, D. et al. Androgen-driven prostate epithelial cell proliferation and differentiation in vivo involve the regulation of p27. Mol. Endocrinol. 15, 765–782 (2001).
Berger, R. et al. Androgen-induced differentiation and tumorigenicity of human prostate epithelial cells. Cancer Res. 64, 8867–8875 (2004). This paper showed that the androgen receptor, which triggers reduced prostate cell growth in vitro upon androgen stimulation, also promotes tumorigenesis in immortalized prostate epithelia following orthotopic injection.
Sicinski, P. et al. Cyclin D1 provides a link between development and oncogenesis in the retina and breast. Cell 82, 621–630 (1995).
Fantl, V., Stamp, G., Andrews, A., Rosewell, I. & Dickson, C. Mice lacking cyclin D1 are small and show defects in eye and mammary gland development. Genes Dev. 9, 2364–2372 (1995).
Chodosh, L. A. The reciprocal dance between cancer and development. N. Engl. J. Med. 347, 134–136 (2002).
Gilliland, D. G. & Griffin, J. D. Role of FLT3 in leukemia. Curr. Opin. Hematol. 9, 274–281 (2002).
Stirewalt, D. L. & Radich, J. P. The role of FLT3 in haematopoietic malignancies. Nature Rev. Cancer 3, 650–665 (2003).
Kumar, V. et al. Functional domains of the human estrogen receptor. Cell 51, 941–951 (1987).
Clarke, R. B., Anderson, E. & Howell, A. Steroid receptors in human breast cancer. Trends Endocrinol. Metab. 15, 316–323 (2004).
Anzick, S. L. et al. AIB1, a steroid receptor coactivator amplified in breast and ovarian cancer. Science 277, 965–968 (1997).
Tanner, M. M. et al. Frequent amplification of chromosomal region 20q12-q13 in ovarian cancer. Clin. Cancer Res. 6, 1833–1839 (2000).
Bingle, C. D. Thyroid transcription factor-1. Int. J. Biochem. Cell Biol. 29, 1471–1473 (1997).
Stenhouse, G., Fyfe, N., King, G., Chapman, A. & Kerr, K. M. Thyroid transcription factor 1 in pulmonary adenocarcinoma. J. Clin. Pathol. 57, 383–387 (2004).
Byrd-Gloster, A. L. et al. Differential expression of thyroid transcription factor 1 in small cell lung carcinoma and Merkel cell tumor. Hum. Pathol. 31, 58–62 (2000).
Hecht, J. L., Pinkus, J. L., Weinstein, L. J. & Pinkus, G. S. The value of thyroid transcription factor-1 in cytologic preparations as a marker for metastatic adenocarcinoma of lung origin. Am. J. Clin. Pathol. 116, 483–488 (2001).
Freund, J. N., Domon-Dell, C., Kedinger, M. & Duluc, I. The Cdx-1 and Cdx-2 homeobox genes in the intestine. Biochem. Cell Biol. 76, 957–969 (1998).
Soubeyran, P. et al. Homeobox gene Cdx1 regulates Ras, Rho and PI3 kinase pathways leading to transformation and tumorigenesis of intestinal epithelial cells. Oncogene 20, 4180–4187 (2001).
de la Chapelle, A. Genetic predisposition to colorectal cancer. Nature Rev. Cancer 4, 769–780 (2004).
Classon, M. & Harlow, E. The retinoblastoma tumour suppressor in development and cancer. Nature Rev. Cancer 2, 910–917 (2002).
Nishi, T. & Saya, H. Neurofibromatosis type 1 (NF1) gene: implication in neuroectodermal differentiation and genesis of brain tumors. Cancer Metastasis Rev. 10, 301–310 (1991).
Salti, G. I. et al. Micropthalmia transcription factor: a new prognostic marker in intermediate-thickness cutaneous malignant melanoma. Cancer Res. 60, 5012–5016 (2000).
Birtle, A. J., Freeman, A., Masters, J. R., Payne, H. A. & Harland, S. J. Clinical features of patients who present with metastatic prostate carcinoma and serum prostate-specific antigen (PSA) levels < 10 ng/mL: the 'PSA negative' patients. Cancer 98, 2362–2367 (2003).
Hainsworth, J. D. & Greco, F. A. Poorly differentiated carcinoma and poorly differentiated adenocarcinoma of unknown primary tumor site. Semin. Oncol. 20, 279–286 (1993).
Ramaswamy, S. et al. Multiclass cancer diagnosis using tumor gene expression signatures. Proc. Natl Acad. Sci. USA 98, 15149–15154 (2001). This paper showed how gene-expression profiles could classify tumours largely on the basis of lineage-differentiation signatures; however, poorly differentiated cancers were not as easily classified by this approach.
Zhang, M. & Rosen, J. M. Stem cells in the etiology and treatment of cancer. Curr. Opin. Genet. Dev. 16, 60–64 (2006).
Kulesa, P. M. et al. Reprogramming metastatic melanoma cells to assume a neural crest cell-like phenotype in an embryonic microenvironment. Proc. Natl Acad. Sci. USA 103, 3752–3757 (2006).
Lu, J. et al. MicroRNA expression profiles classify human cancers. Nature 435, 834–838 (2005). This paper showed that miRNA profiles could classify tumours across multiple lineages, regardless of their differentiation patterns.
Kroll, E. S., Hyland, K. M., Hieter, P. & Li, J. J. Establishing genetic interactions by a synthetic dosage lethality phenotype. Genetics 143, 95–102 (1996).
Kaelin, W. G. The concept of synthetic lethality in the context of anticancer therapy. Nature Rev. Cancer 5, 689–698 (2005).
Hartman, J. L., Garvik, B. & Hartwell, L. Principles for the buffering of genetic variation. Science 291, 1001–1004 (2001).
Kaelin, W. G. Choosing anticancer drug targets in the postgenomic era. J. Clin. Invest. 104, 1503–1506 (1999).
Cairncross, J. G. et al. Specific genetic predictors of chemotherapeutic response and survival in patients with anaplastic oligodendrogliomas. J. Natl Cancer Inst. 90, 1473–1479 (1998).
Sawyers, C. L. Finding the next Gleevec: FLT3 targeted kinase inhibitor therapy for acute myeloid leukemia. Cancer Cell 1, 413–415 (2002).
Tamm, I., Dorken, B. & Hartmann, G. Antisense therapy in oncology: new hope for an old idea? Lancet 358, 489–497 (2001).
Morris, M. J. et al. Phase I trial of BCL-2 antisense oligonucleotide (G3139) administered by continuous intravenous infusion in patients with advanced cancer. Clin. Cancer Res. 8, 679–683 (2002).
Nahta, R. & Esteva, F. J. Bcl-2 antisense oligonucleotides: a potential novel strategy for the treatment of breast cancer. Semin. Oncol. 30, 143–149 (2003).
Genasense FDA review team. Genasense (Oblimersen) for metastatic melanoma [online], (2004).
Wang, J. C. & Dick, J. E. Cancer stem cells: lessons from leukemia. Trends Cell Biol. 15, 494–501 (2005).
Dean, M., Fojo, T. & Bates, S. Tumour stem cells and drug resistance. Nature Rev. Cancer 5, 275–284 (2005).
Garraway, L. A. & Sellers, W. R. Array-based approaches to cancer genome analysis. Drug Discov. Today 2, 171–177 (2005).
Engle, L. J., Simpson, C. L. & Landers, J. E. Using high-throughput SNP technologies to study cancer. Oncogene 25, 1594–1601 (2006).
Beroukhim, R. et al. Inferring loss-of-heterozygosity from tumor-only samples using high-density oligonucleotide SNP arrays. PLoS Comput. Biol. 2, e41 (2006).
Zhao, X. et al. An integrated view of copy number and allelic alterations in the cancer genome using single nucleotide polymorphism arrays. Cancer Res. 64, 3060–3071 (2004).
Jiang, J. et al. Identifying and characterizing a novel activating mutation of the FLT3 tyrosine kinase in AML. Blood 104, 1855–1858 (2004).
Acknowledgements
This work was supported by grants from the US National Cancer Institute to L.A.G. and W.R.S.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
W.R.S. is employed by the Novartis Institutes of Biomedical Research Inc., Cambridge, USA.
Related links
Related links
DATABASES
National Cancer Institute
gastrointestinal stromal tumours
FURTHER INFORMATION
Glossary
- p16–CDK4–RB pathway
-
A vital cell-cycle checkpoint that operates in mammalian cells. The p16 protein (encoded by CDKN2A) is a well-characterized cell-cycle inhibitor, and the RB (Retinoblastoma) protein was one of the first tumour suppressors to be described.
- Synthetic dosage lethality
-
A genetic interaction in which overexpression or activation of one gene or pathway becomes lethal to the cell when a second (normally non-lethal) mutation is also present.
Rights and permissions
About this article
Cite this article
Garraway, L., Sellers, W. Lineage dependency and lineage-survival oncogenes in human cancer. Nat Rev Cancer 6, 593–602 (2006). https://doi.org/10.1038/nrc1947
Issue Date:
DOI: https://doi.org/10.1038/nrc1947
This article is cited by
-
Mapping the landscape of genetic dependencies in chordoma
Nature Communications (2023)
-
Temporal and spatial stability of the EM/PM molecular subtypes in adult diffuse glioma
Frontiers of Medicine (2023)
-
A unique hyperdynamic dimer interface permits small molecule perturbation of the melanoma oncoprotein MITF for melanoma therapy
Cell Research (2023)
-
SOX11 regulates SWI/SNF complex components as member of the adrenergic neuroblastoma core regulatory circuitry
Nature Communications (2023)
-
PITX2C increases the stemness features of hepatocellular carcinoma cells by up-regulating key developmental factors in liver progenitor
Journal of Experimental & Clinical Cancer Research (2022)