Key Points
-
Identifying the cell of origin can uncover the initiating and subsequent genetic changes that occur as cells progress from preneoplastic lesions to tumours.
-
Retinoblastoma is a good tumour model for identifying the cell of origin, because retinal development is well understood and the initiating genetic lesion (loss of RB) is well characterized.
-
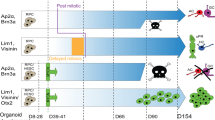
Models of retinoblastoma differ depending on when RB is thought to act and on the response of the cell of origin to RB loss.
-
Additional mutations, beyond RB disruption, must occur for retinoblastoma to develop. These could occur in genes that regulate programmes of cell death or cell differentiation. New mouse models support the latter possibility.
-
Improved animal models could help to identify the retinoblastoma cell of origin, and explain why retinal cells are especially susceptible to transformation following RB disruption.
Abstract
The cellular effects of the genetic defects associated with tumorigenesis are context dependent. To better understand the reasons that different cell types require distinct combinations of mutations to form tumours, it is essential to identify and characterize a tumour's 'cell of origin'. Retinoblastoma, a rare childhood cancer of the retina that is caused by RB inactivation, is a good model in which to search for a tumour cell of origin, because retinal development is well understood and the initiating genetic lesion is well characterized. Identifying the cell of origin for this tumour would advance our understanding of how cellular context affects the requirement of specific mutations for cancer initiation and progression.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Friend, S. H. et al. A human DNA segment with properties of the gene that predisposes to retinoblastoma and osteosarcoma. Nature 323, 643–646 (1986).
Goodrich, L. V., Milenkovic, L., Higgins, K. M. & Scott, M. P. Altered neural cell fates and medulloblastoma in mouse patched mutants. Science 277, 1109–1113 (1997). The first study to show that deregulation of the Hedgehog pathway could lead to tumours in the developing cerebellum. Provides an excellent example of the connection between processes important for development and tumorigenesis.
Goodrich, L. V. & Scott, M. P. Hedgehog and patched in neural development and disease. Neuron 21, 1243–1257 (1998).
Wechsler-Reya, R. J. & Scott, M. P. Control of neuronal precursor proliferation in the cerebellum by Sonic Hedgehog. Neuron 22, 103–114 (1999).
Goldowitz, D. & Hamre, K. The cells and molecules that make a cerebellum. Trends Neurosci. 21, 375–382 (1998).
Dakubo, G. D. et al. Retinal ganglion cell-derived sonic hedgehog signaling is required for optic disc and stalk neuroepithelial cell development. Development 130, 2967–2980 (2003).
Jensen, A. M. & Wallace, V. A. Expression of Sonic hedgehog and its putative role as a precursor cell mitogen in the developing mouse retina. Development 124, 363–371 (1997).
Taylor, M. D., Mainprize, T. G. & Rutka, J. T. Molecular insight into medulloblastoma and central nervous system primitive neuroectodermal tumor biology from hereditary syndromes: a review. Neurosurgery 47, 888–901 (2000).
Chen, D. et al. Cell-specific effects of RB or RB/p107 loss on retinal development implicate an intrinsically death-resistant cell-of-origin in retinoblastoma. Cancer Cell 5, 539–551 (2004). Detailed analysis of the defects in RB/p107-deficient mouse retinae, which provided the first evidence that cells expressing markers of differentiated cell types proliferate but only a subset undergo apoptosis. These data support both the transistion cell of origin model and the idea that post-RB mutations impair differentiation not death.
MacPherson, D. et al. Cell type-specific effects of Rb deletion in the murine retina. Genes Dev. 18, 1681–1694 (2004). Shows that inactivating RB and the third family member, p130, also causes retinoblastoma.
Zhang, J., Schweers, B. & Dyer, M. A. The first knockout mouse model of retinoblastoma. Cell Cycle 3, 952–959 (2004). A Chx10 –Cre transgene was used to drive mosaic RB or RB and p53 inactivation on a p107 -null genetic background. Currently, these are two of the excellent preclinical models of retinoblastoma available for therapeutic studies.
Marino, S., Vooijs, M., van Der Gulden, H., Jonkers, J. & Berns, A. Induction of medulloblastomas in p53-null mutant mice by somatic inactivation of Rb in the external granular layer cells of the cerebellum. Genes Dev. 14, 994–1004 (2000).
DiCiommo, D., Gallie, B. L. & Bremner, R. Retinoblastoma: the disease, gene and protein provide critical leads to understand cancer. Semin. Cancer Biol. 10, 255–269 (2000).
Bremner, R. & Balmain, A. Genetic changes in skin tumor progression: correlation between presence of a mutant ras gene and loss of heterozygosity on mouse chromosome 7. Cell 61, 407–417 (1990).
Bremner, R., Kemp, C. J. & Balmain, A. Induction of different genetic changes by different classes of chemical carcinogens during progression of mouse skin tumors. Mol. Carcinog. 11, 90–97 (1994).
Romer, J. T. et al. Suppression of the Shh pathway using a small molecule inhibitor eliminates medulloblsatoma in Ptc1+/−, p53−/− mice. Cancer Cell 6, 229–240 (2004).
Dyer, M. A. Mouse models of childhood tumors of the nervous system. J. Clin. Pathol. 57, 561–576 (2004).
Classon, M. & Harlow, E. The retinoblastoma tumour suppressor in development and cancer. Nature Rev. Cancer 2, 910–917 (2002).
Classon, M. & Dyson, N. p107 and p130: versatile proteins with interesting pockets. Exp. Cell Res. 264, 135–147 (2001).
Jacks, T. et al. Effects of an Rb mutation in the mouse. Nature 359, 295–300 (1992).
Clarke, A. R. et al. Requirement for a functional Rb-1 gene in murine development. Nature 359, 328–330 (1992).
Maandag, E. C. et al. Developmental rescue of an embryonic-lethal mutation in the retinoblastoma gene in chimeric mice. EMBO J. 13, 4260–4268 (1994).
Lee, E. Y. et al. Mice deficient for Rb are nonviable and show defects in neurogenesis and haematopoiesis. Nature 359, 288–294 (1992).
Robanus-Maandag, E. et al. p107 is a suppressor of retinoblastoma development in pRb-deficient mice. Genes Dev. 12, 1599–1609 (1998). Using chimeric mice, this was the first evidence that loss of p107 combined with loss of RB could lead to retinoblastoma in mice. These studies laid the foundation for subsequent conditional-knockout mouse studies.
Sage, J., Miller, A. L., Perez-Mancera, P. A., Wysocki, J. M. & Jacks, T. Acute mutation of retinoblastoma gene function is sufficient for cell cycle re-entry. Nature 424, 223–228 (2003).
Dyer, M. A. & Cepko, C. L. Regulating proliferation during retinal development. Nature Rev. Neurosci. 2, 333–342 (2001).
Livesey, F. J. & Cepko, C. L. Vertebrate neural cell-fate determination: lessons from the retina. Nature Rev. Neurosci. 2, 109–118 (2001).
Zhu, X. et al. Mechanisms of loss of heterozygosity in retinoblastoma. Cytogenet. Cell Genet. 59, 248–252 (1992).
Harbour, J. W. Molecular basis of low-penetrance retinoblastoma. Arch. Ophthalmol. 119, 1699–1704 (2001).
Zhang, J. et al. Rb regulates proliferation and rod photoreceptor development in the mouse retina. Nature Genet. 36, 351–360 (2004). The first analysis of the role of RB in retinal progenitor cell proliferation and retinal development in the mouse. RB was found to have distinct roles in rod development and retinal progenitor cell proliferation.
Young, R. W. Cell proliferation during postnatal development of the retina in the mouse. Brain Res. 353, 229–239 (1985).
Young, R. W. Cell differentiation in the retina of the mouse. Anat. Rec. 212, 199–205 (1985).
Cepko, C. L., Austin, C. P., Yang, X., Alexiades, M. & Ezzeddine, D. Cell fate determination in the vertebrate retina. Proc. Natl Acad. Sci. USA 93, 589–595 (1996).
Belliveau, M. J. & Cepko, C. L. Extrinsic and intrinsic factors control the genesis of amacrine and cone cells in the rat retina. Development 126, 555–566 (1999).
Belliveau, M. J., Young, T. L. & Cepko, C. L. Late retinal progenitor cells show intrinsic limitations in the production of cell types and the kinetics of opsin synthesis. J. Neurosci. 20, 2247–2254 (2000).
Alexiades, M. R. & Cepko, C. L. Subsets of retinal progenitors display temporally regulated and distinct biases in the fates of their progeny. Development 124, 1119–1131 (1997).
Ezzeddine, Z. D., Yang, X., DeChiara, T., Yancopoulos, G. & Cepko, C. L. Postmitotic cells fated to become rod photoreceptors can be respecified by CNTF treatment of the retina. Development 124, 1055–1067 (1997).
Dyer, M. A. Regulation of proliferation, cell fate specification and differentiation by the homeodomain proteins Prox1, Six3, and Chx10 in the developing retina. Cell Cycle 2, 350–357 (2003).
Dyer, M. A., Livesey, F. J., Cepko, C. L. & Oliver, G. Prox1 function controls progenitor cell proliferation and horizontal cell genesis in the mammalian retina. Nature Genet. 34, 53–58 (2003).
Donovan, S. L. & Dyer, M. A. Regulation of proliferation in the developing central nervous system. Sem. Cell Dev. Biol (in the press).
Dyer, M. A. & Cepko, C. L. p27Kip1 and p57Kip2 regulate proliferation in distinct retinal progenitor cell populations. J. Neurosci. 21, 4259–4271 (2001).
Blackshaw, S. et al. Genomic analysis of mouse retinal development. PLoS Biol. 2, E247 (2004).
Blackshaw, S., Fraioli, R. E., Furukawa, T. & Cepko, C. L. Comprehensive analysis of photoreceptor gene expression and the identification of candidate retinal disease genes. Cell 107, 579–589 (2001).
Blackshaw, S. et al. MicroSAGE is highly representative and reproducible but reveals major differences in gene expression among samples obtained from similar tissues. Genome Biol. 4, R17 (2003).
Nork, T. M., Schwartz, T. L., Doshi, H. M. & Millecchia, L. L. Retinoblastoma. Cell of origin. Arch. Ophthalmol. 113, 791–802 (1995).
Gallie, B. L., Campbell, C., Devlin, H., Duckett, A. & Squire, J. A. Developmental basis of retinal-specific induction of cancer by RB mutation. Cancer Res. 59, 1731s–1735s (1999).
Zheng, L. & Lee, W. H. Retinoblastoma tumor suppressor and genome stability. Adv. Cancer Res. 85, 13–50 (2002).
Bremner, R., Chen, D., Pacal, M., Livne–Bar, I. & Agochiya, M. The Rb protein family in retinal development and retinoblatoma: New insights from new mouse models. Dev. Neurosci. (in the press).
Hernando, E. et al. Rb inactivation promotes genomic instability by uncoupling cell cycle progression from mitotic control. Nature 430, 797–802 (2004).
Rowan, S. & Cepko, C. L. Genetic analysis of the homeodomain transcription factor Chx10 in the retina using a novel multifunctional BAC transgenic mouse reporter. Dev. Biol. 271, 388–402 (2004).
Liu, I. S. et al. Developmental expression of a novel murine homeobox gene (Chx10): evidence for roles in determination of the neuroretina and inner nuclear layer. Neuron 13, 377–393 (1994).
Liu, W. et al. All Brn3 genes can promote retinal ganglion cell differentiation in the chick. Development 127, 3237–3247 (2000).
Marquardt, T. et al. Pax6 is required for the multipotent state of retinal progenitor cells. Cell 105, 43–55 (2001).
Xu, P. X. et al. Regulation of Pax6 expression is conserved between mice and flies. Development 126, 383–395 (1999).
Furukawa, T., Morrow, E. M. & Cepko, C. L. Crx, a novel otx-like homeobox gene, shows photoreceptor-specific expression and regulates photoreceptor differentiation. Cell 91, 531–541 (1997).
Knudson, A. Mutation and cancer: statistical study of retinoblastoma. PNAS 68, 820–823 (1971).
Cavenee, W. K. et al. Genetic origin of mutations predisposing to retinoblastoma. Science 228, 501–503 (1985).
Chen, D. et al. Genomic amplification in retinoblastoma narrowed to 0.6 megabase on chromosome 6p containing a kinesin-like gene, RBKIN. Cancer Res. 62, 967–971 (2002).
Herzog, S. et al. Marked differences in unilateral isolated retinoblastomas from young and older children studied by comparative genomic hybridization. Hum. Genet. 108, 98–104 (2001).
Lillington, D. M. et al. Comparative genomic hybridization of 49 primary retinoblastoma tumors identifies chromosomal regions associated with histopathology, progression, and patient outcome. Genes Chromosom. Cancer 36, 121–128 (2003).
Mairal, A. et al. Detection of chromosome imbalances in retinoblastoma by parallel karyotype and CGH analyses. Genes Chromosom. Cancer 28, 370–379 (2000).
O'Brien, J. M. et al. A transgenic mouse model for trilateral retinoblastoma. Arch. Ophthalmol. 108, 1145–1151 (1990).
Windle, J. J. et al. Retinoblastoma in transgenic mice. Nature 343, 665–669 (1990).
Donovan, S. L. & Dyer, M. A. Developmental defects in Rb-deficient retinae. Vision Res. 44, 3323–3333 (2004).
Armitage, P. & Doll, R. The age distribution of cancer and a multi-stage theory of carcinogenesis. Br. J. Cancer 8, 1–12 (1954).
Renan, M. J. How many mutations are required for tumorigenesis? Implications from human cancer data. Mol. Carcinog. 7, 139–146 (1993).
Hanahan, D. & Weinberg, R. A. The hallmarks of cancer. Cell 100, 57–70 (2000).
Lipinski, M. M. et al. Cell-autonomous and non-cell-autonomous functions of the Rb tumor suppressor in developing central nervous system. EMBO J. 20, 3402–3413 (2001).
MacPherson, D. et al. Conditional mutation of Rb causes cell cycle defects without apoptosis in the central nervous system. Mol. Cell. Biol. 23, 1044–1053 (2003).
Wu, L. et al. Extra-embryonic function of Rb is essential for embryonic development and viability. Nature 421, 942–947 (2003).
Maandag, E. C. et al. Developmental rescue of an embryonic-lethal mutation in the retinoblastoma gene in chimeric mice. EMBO J. 13, 4260–4268 (1994).
Ferguson, K. L. et al. Telencephalon-specific Rb knockouts reveal enhanced neurogenesis, survival and abnormal cortical development. EMBO J. 21, 3337–3346 (2002).
Muller, H. et al. E2Fs regulate the expression of genes involved in differentiation, development, proliferation, and apoptosis. Genes Dev. 15, 267–285 (2001).
Marino, S., Hoogervoorst, D., Brandner, S. & Berns, A. Rb and p107 are required for normal cerebellar development and granule cell survival but not for Purkinje cell persistence. Development 130, 3359–3368 (2003).
Jeon, C. J., Strettoi, E. & Masland, R. H. The major cell populations of the mouse retina. J. Neurosci. 18, 8936–8946 (1998).
Cayouette, M. & Raff, M. The orientation of cell division influences cell-fate choice in the developing mammalian retina. Development 130, 2329–2339 (2003).
Burmeister, M. et al. Ocular retardation mouse caused by Chx10 homeobox null allele: impaired retinal progenitor proliferation and bipolar cell differentiation. Nature Genet. 12, 376–384 (1996).
Belecky-Adams, T. et al. Pax-6, Prox 1, and Chx10 homeobox gene expression correlates with phenotypic fate of retinal precursor cells. Invest. Ophthalmol. Vis. Sci. 38, 1293–1303 (1997).
de Melo, J., Qiu, X., Du, G., Cristante, L. & Eisenstat, D. D. Dlx1, Dlx2, Pax6, Brn3b, and Chx10 homeobox gene expression defines the retinal ganglion and inner nuclear layers of the developing and adult mouse retina. J. Comp. Neurol. 461, 187–204 (2003).
Liu, W., Mo, Z. & Xiang, M. The Ath5 proneural genes function upstream of Brn3 POU domain transcription factor genes to promote retinal ganglion cell development. Proc. Natl Acad. Sci. USA 98, 1649–1654 (2001).
Acknowledgements
Funding for work on RB in the authors' laboratories is supported by grants from the National Institutes of Health, Research to Prevent Blindness, the National Science Foundation and the American Cancer Society (M.A.D.), and the Canadian Institutes of Health Research (R.B.). M.A.D. is a Pew Scholar in Biomedical Sciences.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Related links
DATABASES
Entrez Gene
National Cancer Institute
FURTHER INFORMATION
Glossary
- ECTOPIC CELL DIVISION
-
Cell division that would not normally occur during development or in the adult retina. Ectopic cell division does not necessarily refer to cancer cell cycles.
- SYNTAXIN
-
Nervous-system-specific proteins implicated in the docking of synaptic vesicles.
- Cre RETROVIRAL SYSTEM
-
By using genetically engineered mice with genes containing LoxP sites, retroviral infection can lead to gene inactivation in individual retinal progenitor cells in vivo.
Rights and permissions
About this article
Cite this article
Dyer, M., Bremner, R. The search for the retinoblastoma cell of origin. Nat Rev Cancer 5, 91–101 (2005). https://doi.org/10.1038/nrc1545
Issue Date:
DOI: https://doi.org/10.1038/nrc1545
This article is cited by
-
The telomere complex and the origin of the cancer stem cell
Biomarker Research (2021)
-
Single-cell transcriptome profiling reveals intratumoural heterogeneity and malignant progression in retinoblastoma
Cell Death & Disease (2021)
-
Giant Y79 retinoblastoma cells contain functionally active T-type calcium channels
Pflügers Archiv - European Journal of Physiology (2021)
-
Targeting tyrosine kinases for treatment of ocular tumors
Archives of Pharmacal Research (2019)
-
Heterogeneity in retinoblastoma: a tale of molecules and models
Clinical and Translational Medicine (2017)