Abstract
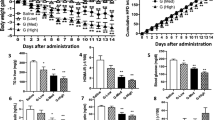
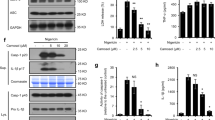
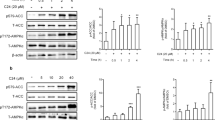
Adipocyte fatty-acid-binding protein, aP2 (FABP4) is expressed in adipocytes and macrophages, and integrates inflammatory and metabolic responses. Studies in aP2-deficient mice have shown that this lipid chaperone has a significant role in several aspects of metabolic syndrome, including type 2 diabetes and atherosclerosis. Here we demonstrate that an orally active small-molecule inhibitor of aP2 is an effective therapeutic agent against severe atherosclerosis and type 2 diabetes in mouse models. In macrophage and adipocyte cell lines with or without aP2, we also show the target specificity of this chemical intervention and its mechanisms of action on metabolic and inflammatory pathways. Our findings demonstrate that targeting aP2 with small-molecule inhibitors is possible and can lead to a new class of powerful therapeutic agents to prevent and treat metabolic diseases such as type 2 diabetes and atherosclerosis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Hotamisligil, G. S. Inflammation and metabolic disorders. Nature 444, 860–867 (2006)
Hertzel, A. V. & Bernlohr, D. A. The mammalian fatty acid-binding protein multigene family: molecular and genetic insights into function. Trends Endocrinol. Metab. 11, 175–180 (2000)
Hunt, C. R., Ro, J. H., Dobson, D. E., Min, H. Y. & Spiegelman, B. M. Adipocyte P2 gene: developmental expression and homology of 5′-flanking sequences among fat cell-specific genes. Proc. Natl Acad. Sci. USA 83, 3786–3790 (1986)
Melki, S. A. & Abumrad, N. A. Expression of the adipocyte fatty acid-binding protein in streptozotocin-diabetes: effects of insulin deficiency and supplementation. J. Lipid Res. 34, 1527–1534 (1993)
Distel, R. J., Robinson, G. S. & Spiegelman, B. M. Fatty acid regulation of gene expression. Transcriptional and post-transcriptional mechanisms. J. Biol. Chem. 267, 5937–5941 (1992)
Hotamisligil, G. S. et al. Uncoupling of obesity from insulin resistance through a targeted mutation in aP2, the adipocyte fatty acid binding protein. Science 274, 1377–1379 (1996)
Uysal, K. T., Scheja, L., Wiesbrock, S. M., Bonner-Weir, S. & Hotamisligil, G. S. Improved glucose and lipid metabolism in genetically obese mice lacking aP2. Endocrinology 141, 3388–3396 (2000)
Scheja, L. et al. Altered insulin secretion associated with reduced lipolytic efficiency in aP2-/- mice. Diabetes 48, 1987–1994 (1999)
Makowski, L. et al. Lack of macrophage fatty-acid-binding protein aP2 protects mice deficient in apolipoprotein E against atherosclerosis. Nature Med. 7, 699–705 (2001)
Kazemi, M. R., McDonald, C. M., Shigenaga, J. K., Grunfeld, C. & Feingold, K. R. Adipocyte fatty acid-binding protein expression and lipid accumulation are increased during activation of murine macrophages by toll-like receptor agonists. Arterioscler. Thromb. Vasc. Biol. 25, 1220–1224 (2005)
Fu, Y., Luo, N. & Lopes-Virella, M. F. Oxidized LDL induces the expression of ALBP/aP2 mRNA and protein in human THP-1 macrophages. J. Lipid Res. 41, 2017–2023 (2000)
Pelton, P. D., Zhou, L., Demarest, K. T. & Burris, T. P. PPARγ activation induces the expression of the adipocyte fatty acid binding protein gene in human monocytes. Biochem. Biophys. Res. Commun. 261, 456–458 (1999)
Boord, J. B. et al. Adipocyte fatty acid-binding protein, aP2, alters late atherosclerotic lesion formation in severe hypercholesterolemia. Arterioscler. Thromb. Vasc. Biol. 22, 1686–1691 (2002)
Sulsky, R. et al. Potent and selective biphenyl azole inhibitors of adipocyte fatty acid binding protein (aFABP). Bioorg. Med. Chem. Lett. (in the press)
Makowski, L., Brittingham, K. C., Reynolds, J. M., Suttles, J. & Hotamisligil, G. S. The fatty acid-binding protein, aP2, coordinates macrophage cholesterol trafficking and inflammatory activity. Macrophage expression of aP2 impacts peroxisome proliferator-activated receptor γ and IκB kinase activities. J. Biol. Chem. 280, 12888–12895 (2005)
Hirosumi, J. et al. A central role for JNK in obesity and insulin resistance. Nature 420, 333–336 (2002)
Tuncman, G. et al. Functional in vivo interactions between JNK1 and JNK2 isoforms in obesity and insulin resistance. Proc. Natl Acad. Sci. USA 103, 10741–10746 (2006)
Maeda, K. et al. Adipocyte/macrophage fatty acid binding proteins control integrated metabolic responses in obesity and diabetes. Cell Metab. 1, 107–119 (2005)
Cao, H. et al. Regulation of metabolic responses by adipocyte/macrophage fatty acid-binding proteins in leptin-deficient mice. Diabetes 55, 1915–1922 (2006)
Llaverias, G. et al. Atorvastatin reduces CD68, FABP4, and HBP expression in oxLDL-treated human macrophages. Biochem. Biophys. Res. Commun. 318, 265–274 (2004)
Fu, Y., Luo, N., Lopes-Virella, M. F. & Garvey, W. T. The adipocyte lipid binding protein (ALBP/aP2) gene facilitates foam cell formation in human THP-1 macrophages. Atherosclerosis 165, 259–269 (2002)
Fisher, R. M. et al. Fatty acid binding protein expression in different adipose tissue depots from lean and obese individuals. Diabetologia 44, 1268–1273 (2001)
Tuncman, G. et al. A genetic variant at the fatty acid-binding protein aP2 locus reduces the risk for hypertriglyceridemia, type 2 diabetes, and cardiovascular disease. Proc. Natl Acad. Sci. USA 103, 6970–6975 (2006)
Sethi, J. K. et al. Characterisation of receptor-specific TNFα functions in adipocyte cell lines lacking type 1 and 2 TNF receptors. FEBS Lett. 469, 77–82 (2000)
Babaev, V. R., Patel, M. B., Semenkovich, C. F., Fazio, S. & Linton, M. F. Macrophage lipoprotein lipase promotes foam cell formation and atherosclerosis in low density lipoprotein receptor-deficient mice. J. Biol. Chem. 275, 26293–26299 (2000)
Kim, J. K. et al. Redistribution of substrates to adipose tissue promotes obesity in mice with selective insulin resistance in muscle. J. Clin. Invest. 105, 1791–1797 (2000)
Blasi, E. et al. Selective immortalization of murine macrophages from fresh bone marrow by a raf/myc recombinant murine retrovirus. Nature 318, 667–670 (1985)
Bligh, E. G. & Dyer, W. J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37, 911–917 (1959)
Acknowledgements
This work was supported in part by grants from the NIH and the American Diabetes Association. M.F. is supported by a JSPS Postdoctoral Fellowship for Research Abroad from the Japan Society for the Promotion of Science. G.T. is supported by a fellowship from the Iacocca Foundation.
Author Contributions G.S.H. designed and supervised experiments and analysed data. M.F. designed and performed experiments and analysed data. G.T., C.Z.G., E.V. and K.K. performed experiments. L.M. and G.A. developed cell lines from mice. V.R.B., S.F. and M.F.L. analysed lipoprotein profiles and advised on experiments. R.S., J.A.R. and R.A.P developed the aP2 inhibitor, BMS309403. M.F. and G.S.H wrote the manuscript. All authors discussed the results and commented on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
[COMPETING INTERESTS STATEMENT: R.S., J.A.R. and R.A.P are employed by the Bristol–Myers Squibb (BMS) Pharmaceutical Research Institute, a for-profit company developing drugs to treat the diseases in question. BMS developed the aP2 inhibitor, BMS309403, used in this study. G.S.H. has joint intellectual property on the use of aP2 inhibitor in diabetes and atherosclerosis.]
Supplementary information
Supplementary Information
This file contains Supplementary Figures S1-S8 with Legends and Supplementary Tables S1-S3. (PDF 774 kb)
Rights and permissions
About this article
Cite this article
Furuhashi, M., Tuncman, G., Görgün, C. et al. Treatment of diabetes and atherosclerosis by inhibiting fatty-acid-binding protein aP2. Nature 447, 959–965 (2007). https://doi.org/10.1038/nature05844
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature05844
This article is cited by
-
Glomerular expression and urinary excretion of fatty acid-binding protein 4 in IgA nephropathy
Journal of Nephrology (2023)
-
Fatty Acid-Binding Protein 4 is Essential for the Inflammatory and Metabolic Response of Microglia to Lipopolysaccharide
Journal of Neuroimmune Pharmacology (2023)
-
FABP4 activates the JAK2/STAT2 pathway via Rap1a in the homocysteine-induced macrophage inflammatory response in ApoE mice atherosclerosis
Laboratory Investigation (2022)
-
Fatty acid-binding protein 4 is a therapeutic target for septic acute kidney injury by regulating inflammatory response and cell apoptosis
Cell Death & Disease (2022)
-
Metabolic reprogramming from glycolysis to fatty acid uptake and beta-oxidation in platinum-resistant cancer cells
Nature Communications (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.