Abstract
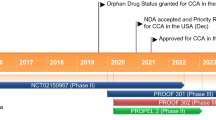
Pemigatinib (PEMAZYRE™), a small molecule inhibitor of fibroblast growth factor receptor (FGFR) 1, FGFR2 and FGFR3, received accelerated approval in April 2020 in the USA for the treatment of adults with previously treated, unresectable, locally advanced or metastatic cholangiocarcinoma and a FGFR2 fusion or other rearrangement, as detected by a US FDA-approved test. Developed by Incyte Corporation, it is the first targeted treatment for cholangiocarcinoma in the USA. The recommended dosage of pemigatinib is 13.5 mg once daily, administered orally with or without food, on days 1–14 of a 21-day cycle until disease progression or unacceptable toxicity. Pemigatinib received orphan designation for the treatment of myeloid/lymphoid neoplasms with eosinophilia and rearrangement of PDGFRA, PDGFRB or FGFR1, or with PCM1-JAK2 in August 2019 in the USA. A regulatory assessment for pemigatinib as a treatment for adults with locally advanced or metastatic cholangiocarcinoma and a FGFR2 fusion or rearrangement that is relapsed or refractory after ≥ 1 line of systemic therapy is underway in the EU. Pemigatinib is also undergoing clinical development in various countries worldwide for use in several other FGFR-driven malignancies (e.g. solid tumour, urothelial carcinoma). This article summarizes the milestones in the development of pemigatinib leading to this first approval for the treatment of adults with previously treated, unresectable, locally advanced or metastatic cholangiocarcinoma and a FGFR2 fusion or other rearrangement, as detected by a US FDA-approved test.
Similar content being viewed by others
References
Rizvi S, Gores GJ. Pathogenesis, diagnosis, and management of cholangiocarcinoma. Gastroenterology. 2013;145(6):1215–29.
US National Library of Medicine. Genetics Home Reference: Cholangiocarcinoma. 2020;2020.
Pellino A, Loupakis F, Cadamuro M, et al. Precision medicine in cholangiocarcinoma. Transl Gastroenterol Hepatol. 2018;3:40.
Incyte Corporation. PEMAZYRE™ (pemigatinib) tablets, for oral use: US prescribing information. 2020. http://www.fda.gov/. Accessed 28 Apr 2020.
Incyte Corporation. FDA approves Incyte’s Pemazyre™ (pemigatinib) as first targeted treatment for adults with previously treated, unresectable locally advanced or metastatic cholangiocarcinoma [media release]. 17 Apr 2020. http://www.incyte.com.
US FDA. FDA grants accelerated approval to pemigatinib for cholangiocarcinoma with an FGFR2 rearrangement or fusion [media release]. 17 Apr 2020. http://www.fda.gov/.
US FDA. Orphan drug designations and approvals. 2019. https://www.accessdata.fda.gov/scripts/opdlisting/oopd/index.cfm. Accessed 08 May 2020.
Incyte Corporation. Incyte and Foundation Medicine announce agreement to develop companion diagnostic for pemigatinib (INCB54828), a selective FGFR inhibitor, in patients with cholangiocarcinoma [media release]. 11 Apr 2018. http://www.incyte.com.
Incyte Corporation. Innovent and Incyte announce strategic collaboration and licensing agreement for three clinical-stage product candidates in China [media release]. 16 Dec 2018. http://www.incyte.com.
Liu PCC, Koblish H, Wu L, et al. INCB054828 (pemigatinib), a potent and selective inhibitor of fibroblast growth factor receptors 1, 2, and 3, displays activity against genetically defined tumor models. PLoS One. 2020;15(4):e0231877.
Subbiah V, Barve M, Iannotti N, et al. FIGHT-101: a phase 1/2 study of pemigatinib, a highly selective fibroblast growth factor receptor (FGFR) inhibitor, as monotherapy and as combination therapy in patients with advanced malignancies [abstract no. A078]. In: AACR-NCI-EORTC International Conference on Molecular Targets and Cancer Therapeutics. 2019.
Kuboki Y, Furukawa M, Takahashi Y, et al. Preliminary results from FIGHT-102: a phase 1 study of pemigatinib in Japanese patients with advanced malignancies [abstract no. P1-156]. Ann Oncol. 2019;30(Suppl 6):vi125.
Necchi A, Pouessel D, Leibowitz-Amit R, et al. Interim results of FIGHT-201, a phase II, open-label, multicenter study of INCB054828 in patients (pts) with metastatic or surgically unresectable urothelial carcinoma (UC) harboring fibroblast growth factor (FGF)/FGF receptor (FGFR) genetic alterations (GA) [abstract no. 900P]. Ann Oncol. 2018;29(Suppl 8):viii319–viii20.
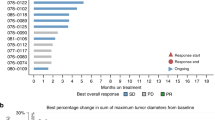
Abou-Alfa GK, Sahai V, Hollebecque A, et al. Pemigatinib for previously treated, locally advanced or metastatic cholangiocarcinoma: a multicentre, open-label, phase 2 study. Lancet Oncol. 2020;21(5):671–84.
Foundation Medicine. Foundation Medicine receives FDA approval for FoundationOne(Rm)CDx as the companion diagnostic for Pemazyre(Tm) (pemigatinib), the first FDA-approved targeted therapy for adults with previously treated locally advanced or metastatic cholangiocarcinoma [media release]. 17 Apr 2020. http://www.foundationmedicine.com.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Conflict of interest
During the peer review process the manufacturer of the agent under review was offered an opportunity to comment on the article. Changes resulting from any comments received were made by the authors on the basis of scientific completeness and accuracy. Sheridan Hoy is a salaried employee of Adis International Ltd/Springer Nature, is responsible for the article content and declares no relevant conflicts of interest.
Additional information
Enhanced material
for this AdisInsight Report can be found at https://doi.org/10.6084/m9.figshare.12355388.
This profile has been extracted and modified from the AdisInsight database. AdisInsight tracks drug development worldwide through the entire development process, from discovery, through pre-clinical and clinical studies to market launch and beyond.
Rights and permissions
About this article
Cite this article
Hoy, S.M. Pemigatinib: First Approval. Drugs 80, 923–929 (2020). https://doi.org/10.1007/s40265-020-01330-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-020-01330-y