Abstract
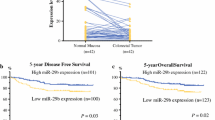
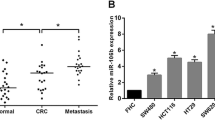
Colorectal cancer (CRC) has a high prevalence and mortality rate. Biomarkers for predicting the recurrence of CRC are not clinically available. This study investigated the role of circulating miR-15b in the prediction of CRC recurrence and the associated mechanism. miR-15b levels in plasma and tissues were measured by real-time PCR. Metastasis suppressor-1 (MTSS1) and Klotho protein expression were detected by Western blot and immunohistochemistry. Invasion and migration of CRC tumor cells were measured by transwell plates. Liver metastasis was established by intraspleen injection of HCT116 cells. Plasma miR-15b levels were significantly higher in CRC patients than in healthy controls, in CRC patients with metastasis than in CRC patients without metastasis, and in CRC patients with recurrence than in CRC patients without recurrence in the 5-year follow-up. miR-15b level in CRC tumors was significantly higher than that in peritumoral tissues. High plasma miR-15b level and negative MTSS1 and Klotho expression in tumor tissues significantly correlated with poor survival. Inhibition of miR-15b activity by adenovirus carrying antimiR-15b sequence significantly increased MTSS1 and Klotho protein expression and subsequently decreased colony formation ability, invasion, and migration of HCT116 cells in vitro and liver metastasis of HCT116 tumors in vivo. In conclusion, high abundance of circulating miR-15b correlated with tumor metastasis, recurrence, and poor patient prognosis through downregulation of MTSS1 and Klotho protein expression.




Similar content being viewed by others
References
Jorgensen ML, Young JM, Solomon MJ. Optimal delivery of colorectal cancer follow-up care: improving patient outcomes. Patient Relat Outcome Meas. 2015;6:127–38.
Siegel R, Ma J, Zhaohui Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29.
Siegel R, DeSantis C, Jemal A. Colorectal cancer statistics, 2014. CA Cancer J Clin. 2014;64:104–17.
Bujko K, Glimelius B, Valentini V, Michalski W, Spalek M. Postoperative chemotherapy in patients with rectal cancer receiving preoperative radio(chemo)therapy: a meta-analysis of randomized trials comparing surgery ± a fluoropyrimidine and surgery + a fluoropyrimidine ± oxaliplatin. Eur J Surg Oncol. 2015;41:713–23.
Buie WD, Attard JA. Follow-up recommendations for colon cancer. Clin Colon Rectal Surg. 2005;18:232–43.
Meyerhardt JA, Mangu PB, Flynn PJ, Korde L, Loprinzi CL, Minsky BD, et al. Follow-up care, surveillance protocol, and secondary prevention measures for survivors of colorectal cancer: American Society of Clinical Oncology clinical practice guideline endorsement. J Clin Oncol. 2013;31:4465–70.
Barrett LW, Sue FS, Wilton SD. Regulation of eukaryotic gene expression by the untranslated gene regions and other non-coding elements. Cell Mol Life Sci. 2012;69:3613–34.
Friedman RC, Farh KK, Burge CB, Bartel DP. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 2009;19:92–105.
Jansson MD, Lund AH. MicroRNA and cancer. Mol Oncol. 2012;6:590–610.
Bertoli G, Cava C, Castiglioni I. MicroRNAs: new biomarkers for diagnosis, prognosis, therapy prediction and therapeutic tools for breast cancer. Theranostics. 2015;5:1122–43.
Hollis M, Nair K, Vyas A, Chaturvedi LS, Gambhir S, Vyas D. MicroRNAs potential utility in colon cancer: early detection, prognosis, and chemosensitivity. World J Gastroenterol. 2015;21:8284–92.
Mostert B, Sieuwerts AM, Martens JW, Sleijfer S. Diagnostic applications of cell-free and circulating tumor cell-associated miRNAs in cancer patients. Expert Rev Mol Diagn. 2011;11:259–75.
Fesler A, Jiang J, Zhai H, Ju J. Circulating microRNA testing for the early diagnosis and follow-up of colorectal cancer patients. Mol Diagn Ther. 2014;18:303–8.
Satzger I, Mattern A, Kuettler U, Weinspach D, Voelker B, Kapp A, et al. MicroRNA-15b represents an independent prognostic parameter and is correlated with tumor cell proliferation and apoptosis in malignant melanoma. Int J Cancer. 2010;126:2553–62.
Kedmi M, Ben-Chetrit N, Körner C, Mancini M, Ben-Moshe NB, Lauriola M, et al. EGF induces microRNAs that target suppressors of cell migration: miR-15b targets MTSS1 in breast cancer. Sci Signal. 2015;8:ra29.
Ota T, Doi K, Fujimoto T, Tanaka Y, Ogawa M, Matsuzaki H, et al. KRAS up-regulates the expression of miR-181a, miR-200c and miR-210 in a three-dimensional-specific manner in DLD-1 colorectal cancer cells. Anticancer Res. 2012;32:2271–5.
Zhang X, Kon T, Wang H, Li F, Huang Q, Rabbani ZN, et al. Enhancement of hypoxia-induced tumor cell death in vitro and radiation therapy in vivo by use of small interfering RNA targeted to hypoxia-inducible factor-1alpha. Cancer Res. 2004;64:8139–42.
Shivapurkar N, Weiner LM, Marshall JL, Madhavan S, Deslattes Mays A, Juhl H, et al. Recurrence of early stage colon cancer predicted by expression pattern of circulating microRNAs. PLoS One. 2014;9:e84686.
Xie F, Ye L, Ta M, Zhang L, Jiang WG. MTSS1: a multifunctional protein and its role in cancer invasion and metastasis. Front Biosci (Schol Ed). 2011;3:621–31.
Kayser G, Csanadi A, Kakanou S, Prasse A, Kassem A, Stickeler E, et al. Downregulation of MTSS1 expression is an independent prognosticator in squamous cell carcinoma of the lung. Br J Cancer. 2015;112:866–73.
Zhang J, Tong Y, Ren L, Li CD. Expression of metastasis suppressor 1 in cervical carcinoma and the clinical significance. Oncol Lett. 2014;8:2145–9.
Zhang S, Qi Q. MTSS1 suppresses cell migration and invasion by targeting CTTN in glioblastoma. J Neurooncol. 2015;121:425–31.
Zhou L, Li J, Shao QQ, Guo JC, Liang ZY, Zhou WX et al. Expression and significances of MTSS1 in pancreatic cancer. Pathol Oncol Res 2015 Jul 22. [Epub ahead of print]
Xie B, Chen J, Liu B, Zhan J. Klotho acts as a tumor suppressor in cancers. Pathol Oncol Res. 2013;19:611–7.
Camilli TC, Xu M, O'Connell MP, Chien B, Frank BP, Subaran S, et al. Loss of Klotho during melanoma progression leads to increased filamin cleavage, increased Wnt5A expression, and enhanced melanoma cell motility. Pigment Cell Melanoma Res. 2011;24:175–86.
Doi S, Zou Y, Togao O, Pastor JV, John GB, Wang L, et al. Klotho inhibits transforming growth factor-beta1 (TGF-beta1) signaling and suppresses renal fibrosis and cancer metastasis in mice. J Biol Chem. 2011;286:8655–65.
Zheng X, Chopp M, Lu Y, Buller B, Jiang F. MiR-15b and miR-152 reduce glioma cell invasion and angiogenesis via NRP-2 and MMP-3. Cancer Lett. 2013;329:146–54.
Zhao Z, Zhang L, Yao Q, Tao Z. miR-15b regulates cisplatin resistance and metastasis by targeting PEBP4 in human lung adenocarcinoma cells. Cancer Gene Ther. 2015;22:108–14.
Xia L, Zhang D, Du R, Pan Y, Zhao L, Sun S, et al. miR-15b and miR-16 modulate multidrug resistance by targeting BCL2 in human gastric cancer cells. Int J Cancer. 2008;123:372–9.
Xia H, Qi Y, Ng SS, Chen X, Chen S, Fang M, et al. MicroRNA-15b regulates cell cycle progression by targeting cyclins in glioma cells. Biochem Biophys Res Commun. 2009;380:205–10.
Acknowledgments
This study was supported by the National 863 Hi-tech Project of China (2007AA021803, 2007AA021901, 2007AA021809, 2007AA021811), the National Natural Science Foundation of China (81272972), National Basic Research Program of China (2010CB833605), Hunan Provincial Science and Technology Department (2010FJ4030), Incubation Program for National Natural Science Funds for Distinguished Young Scholar of Central South University (2010QYZD006), and Open-End Fund for the Valuable and Precision Instruments of Central South University.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
This study was approved by the Ethics Committee for Human Research of Xiangya Hospital. The animal protocol was preapproved by Central South University, and all experiments were performed in accordance to the animal care guidelines of the Chinese Council.
Conflicts of interest
None
Rights and permissions
About this article
Cite this article
Li, J., Chen, Y., Guo, X. et al. Inhibition of miR-15b decreases cell migration and metastasis in colorectal cancer. Tumor Biol. 37, 8765–8773 (2016). https://doi.org/10.1007/s13277-015-4396-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-015-4396-9