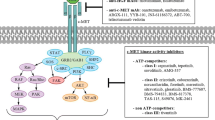
Opinion statement
Lung cancer is the most common malignant neoplasm and constitutes the most common neoplastic cause of death globally. The results of therapies employing standard chemotherapy are unsatisfactory. Currently, efforts are being made to personalize the therapy; numerous clinical studies are being conducted around the world to assess the efficacy and safety of agents directed at molecular targets. One of these molecular targets is the c-MET proto-oncogene, whose primary ligand is hepatocyte growth factor (HGF). C-MET hyperactivity has been observed in numerous neoplasms, including non-small-cell lung carcinoma. Prolonged or continuous activity of the receptor leads to excessive cell proliferation and is related to the development or progression of neoplastic disease. C-MET inhibitors can be classified into three groups: small-molecule tyrosine kinase inhibitors of the c-MET receptor (crizotinib, tivantinib, cabozantinib, foretinib), as well as monoclonal antibodies against c-MET (onartuzumab) and against the HGF ligand (ficlatuzumab, rilotumumab). The efficacy and safety of these agents is assessed both in monotherapy and in combination with other molecularly targeted agents. Furthermore, the toxicity profile of c-MET inhibitors is completely different from that of standard chemotherapy. The best understood c-MET inhibitor used in the treatment of non-small-cell lung carcinoma patients is crizotinib. It is registered for patients with the presence of ALK gene rearrangements after the failure of the first line of treatment based on platinum derivatives. The purpose of this present paper is to present clinical studies that assessed the efficacy and safety of c-MET inhibitors for the treatment of non-small-cell lung carcinoma, as well as current indications for the use of these molecules.
Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
West L, Vidwans SJ, Campbell NP, et al. A novel classification of lung cancer into molecular subtypes. PLoS One. 2012;7:e31906.
Feng Y, Ma CP. MET targeted therapy for lung cancer: clinical development and future directions. Lung Cancer: Targetes and Ther. 2012;3:53–66. This paper clearly presents structure and function of MET receptor as well as its alterations in lung cancer.
Xie Q, Bradley R, Kang L, et al. Hepatocyte growth factor(HGF) autocrine activation predicts sensitivity to MET inhibition in glioblastoma. Proc Natl Acad Sci U S A. 2012;109(2):570–5.
Organ SL, Tsao MS. An overview of the c-MET signaling pathway. Ther Adv Med Oncol. 2011;3(S1):7–19.
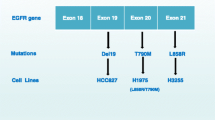
Engelman JA, Zejnuliahu K, Mitsudomi T, et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science. 2007;316:1039–43.
Bean J, Brennan C, Shih JY, et al. MET amplification occurs with or without T790M mutations in EGFR mutant lung tumors with acquired resistance to gefitinib or erlotinib. Proc Natl Acad Sci U S A. 2007;104:20932–7.
Sahrma N, Adjei A. In the clinic: ongoing clinical trials evaluating c-MET-inhibiting drugs. Ther Adv Med Oncol. 2011;3(S1):37–50.
Smyth EC, Sclafani F, Cunningham D. Emerging molecular targets in oncology: clinical potetial of MET/hepatocyte growth-factor. Oncol Targets Ther. 2014;7:1001–14. This paper discuss the role of MET’s dysregulation in different cancers and highlight the potential benefits related to the use of MET/HGF inhibitors.
Ou SI, Kwak EL, Siwak-Tapp C, et al. Activity of Crizotinib (PF02341066), a Dual Mesenchymal-Epithelial Transition (MET) and Anaplastic Lymphoma Kinase (ALK) Inhibitor, in Non-small Cell Lung Cancer Patient with De Novo MET Amplification. J Thorac Oncol. 2011;5:942–6.
Shaw A, Yeap B, Solomon B, et al. Effect of crizotinib on overall survival in patients with advanced non-small-cell lung cancer harbouring ALK gene rearrangement: a retrospective analysis. Lancet Oncol. 2011;12(11):1004–12.
Weickhardt AJ, Camidge DR. The therapeutic potential of anaplastic lymphoma kinase inhibitors in lung cancer: rationale and clinical evidence. Clin Invest. 2011;1:1119–26.
Pfizer. A Study Of Oral PF-02341066, A c-Met/hepatocyte growth factor tyrosine kinase inhibitor, in patients with advanced cancer. Available from: http://www.clinicaltrials.gov/ct2/show/NCT00585195. NLM identifier: NCT00585195. Accessed July 3, 2014.
Pfizer. An investigational drug, PF-02341066, is being studied in patients with advanced non-small cell lung cancer with a specific gene profile involving the anaplastic lymphoma kinase (ALK) gene. Available from: http://www.clinicaltrials.gov/ct2/show/NCT00932451. NLM identifier: NCT00932451. Accessed July 3, 2014.
Camidge R, Bang Y-J, Kwak E, et al. Activity and safety of crizotinb in patients ALK-positive non-small-cell lung cancer: updated results from a phase 1 study. Lancet Oncol. 2012;13:1011–9. This paper describes the results regarding efficacy and safety of crizotinib compared with standard chemotherapy in patients with NSCLC after the fail of first-line treatment. It provides evidence and points out the benefits from use of crizotynib in this setting.
Pfizer. An investigational drug, PF-02341066, is being studied versus standard of care in patients with advanced non-small cell lung cancer with a specific gene profile involving the anaplastic lymphoma kinase (ALK) Gene. Available from: http://www.clinicaltrials.gov/ct2/show/NCT00932893. NLM identifier: NCT00932893. Accessed July 3, 2014.
Shaw A, Kim DW, Nakagawa K, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med. 2013;368:2385–94. This paper describes the result of phase 1 study that was the basis to register crizotinib for the treatment of patients suffering from non-small-cell lung carcinoma with the presence of rearrangements within the ALK gene after the failure of previous treatment.
Pfizer. A Clinical Trial Testing The Efficacy Of Crizotinib Versus Standard Chemotherapy Pemetrexed Plus Cisplatin Or Carboplatin In Patients With ALK Positive Non Squamous Cancer Of The Lung (PROFILE 1014). Available from: http://www.clinicaltrials.gov/ct2/show/NCT01154140. NLM identifier: NCT01154140. Accessed July 3, 2014.
Mok T, Dong-Wan K, Yi-Long W, et al. First-line crizotinib versus pemetrexed–cisplatin or pemetrexed–carboplatin in patients (pts) with advanced ALK-positive non-squamous non-small cell lung cancer (NSCLC): results of a phase III study (PROFILE 1014). J Clin Oncol. 2014;32:5s. Suppl: abstr 8002.
Pfizer. Erlotinib is being studied with or without an investigational drug, PF-02341066, in patients with lung cancer. Available from: http://www.clinicaltrials.gov/ct2/show/NCT00965731. NLM identifier: NCT00965731. Accessed July 3, 2014.
Pfizer. A study of combined C-MET inhibitor and PAN-HER inhibitor (PF-02341066 and PF-00299804) in patients with non- small cell lung cancer. Available from: http://www.clinicaltrials.gov/ct2/show/NCT01121575. NLM identifier: NCT01121575. Accessed July 3, 2014.
Camidge DR, Ou SH, Shapiro G, et al. Efficacy and safety of crizotinib in patients with advanced c-MET-amplified non-small cell lung cancer (NSCLC). J Clin Oncol. 2014;32:5s. Suppl: abstr 8001.
Nishina T, Hirashima T, Sugio K, et al. The effect of CYP2C19 polymorphism on the tolerability of ARQ 197: results from phase I trial in Japanese patients with metastatic solid tumors [abstract]. J Clin Oncol. 2011;29 Suppl:2516.
Sequist LV, von Pawel J, Garmey EG, et al. Randomized phase II study of erlotinib plus tivantinib versus erlotinib plus placebo in previously treated non-small-cell lung cancer. J Clin Oncol. 2011;29(24):3307–15. This paper presents the results of randomized phase II study of erlotinib plus tivantinib versus erlotinib plus placebo in previously treated NSCLC with a detailed analysis of the factors influencing PFS and OS in study population.
Scagliotti GV, Novello S, Schiller JH, et al. Rationale and design of MARQUEE: a phase III, randomized, double-blind study of tivantinib plus erlotinib versus placebo plus erlotinib in previously treated patients with locally advanced or metastatic, nonsquamous, non-small-cell lung cancer. Clin Lung Cancer. 2012;13(5):391–5.
Scagliotti GV, Novello S, von Pawel J. The emerging role of MET/HGF inhibitors in oncology. Cancer Treat Rev. 2013;39(7):793–801.
Kyowa Hakko Kirin Company, Limited. A Phase 3, Randomized, Double-Blinded, Placebo-Controlled Study of ARQ 197 Plus Erlotinib Versus Placebo Plus Erlotinib (ATTENTION). Available from: http://clinicaltrials.gov/ct2/show/NCT01377376. NLM identifier: NCT01377376. Accessed July 3, 2014.
ArQule. Erlotinib Plus Tivantinib (ARQ 197) Versus Single Agent Chemotherapy in Locally Advanced or Metastatic Non-Small Cell Lung Cancer. Available from: http://www.clinicaltrials.gov/ct2/show/NCT01395758. NLM identifier: NCT01395758. Accessed July 3, 2014.
Bristol-Myers Squibb. Multiple ascending dose study of BMS-907351 (XL184) in patients with solid tumors in Japan. Available from: http://www.clinicaltrials.gov/ct2/show/NCT01018745. NLM identifier: NCT01018745. Accessed July 3, 2014.
Padda S, Neal JW, Wakelee HA. MET inhibitor combination therapy in lung cancer. Transl Lung Cancer Res. 2012;1(4):238–53.
Exelixis. A study of XL184 (Cabozantinib) with or without erlotinib in adults with non-small cell lung cancer. NCT00596648. Available from: http://www.clinicaltrials.gov/ct2/show/NCT00596648. NLM identifier: NCT00596648. Accessed July 3, 2014.
Reckamp KL, Frankel PH, Mack PC, et al. Phase II trial of XL184 (cabozantinib) plus erlotinib in patients (pts) with advanced EGFR-mutant non-small cell lung cancer (NSCLC) with progressive disease (PD) on epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor (TKI) therapy: A California Cancer Consortium phase II trial (NCI 9303). J Clin Oncol. 2014;32:5s. Suppl: abstr 8014.
National Cancer Institute. Erlotinib hydrochloride and Cabozantinib-s-Malate alone or in combination as second or third line therapy in treating patients with stage IV Non-Small Cell Lung Cancer. NCT01708954. Available from: http://www.clinicaltrials.gov/ct2/show/NCT01708954. NML identifier: NCT01708954. Accessed August 31, 2014.
Liu L, Shi H, Liu Y, et al. Synergistic effects of foretinib with HER-targeted agents in MET and HER1- or HER2-coactivated tumor cells. Mol Cancer Ther. 2011;10:518–30.
Naing A, Kurzrock R, Adams LM, et al. A comparison of the pharmacokinetics of the anticancer MET inhibitor foretinib free base tablet formulation to bisphosphate salt capsule formulation in patients with solid tumors. Investig New Drugs. 2012;30:327–34.
Eder JP, Shapiro GI, Appleman LJ, et al. A phase I study of foretinib, a multi-targeted inhibitor of c-Met and vascular endothelial growth factor receptor 2. Clin Cancer Res. 2010;16:3507–16.
NCIC Clinical Trials Group. MET/VEGFR2 Inhibitor GSK1363089 and Erlotinib Hydrochloride or Erlotinib Hydrochloride Alone in Treating Patients With Locally Advanced or Metastatic Non-Small Cell Lung Cancer That Has Not Responded to Previous Chemotherapy. Available from: http://www.clinicaltrials.gov/ct2/show/NCT01068587. NLM identifier: NCT01068587. Accessed July 3, 2014.
Yi-Long Wu, James Chih-Hsin Yang, Dong-Wan Kim et al. Safety and efficacy of INC280 in combination with gefitinib in patients with EGFR-mutated, MET-positive NSCLC: A single-arm phase Ib/II study. J Clin Oncol. 2014; 32:5s, Suppl: abstr 8017 The abstract shows promising results of combination therapy: INC280 as a MET inhibitor plus gefitynib in EGFR-mutated, MET-positive NSCLC patients.
Berinstein NL, Morse M, Kaufman H, et al. A phase I study of the safety and immunogenicity of a therapeutic vaccine, DPX-0907 in patients with advanced-stage ovarian, breast, or prostate cancer [abstract]. J Clin Oncol. 2011;29 Suppl:e13050.
Genentech. A study of MetMAb administered to patients with advanced non-small cell lung cancer, in combination with Tarceva (Erlotinib). Available from: http://www.clinicaltrials.gov/ct2/show/NCT00854308. NLM identifier: NCT NCT00854308. Accessed July 3, 2014.
Spigel DR, Ervin TJ, Ramlau R, et al. Randomized phase II trial of Onartuzumab in combination with erlotinib in advanced non-small-cell lung cancer. J Clin Oncol. 2013;31 Suppl abstr 4105. The study resulted in the registration of the immunohistochemical test marking the expression of the MET receptor as a potentially important predictive biomarker.
Genentech. A study of Onartuzumab (MetMAb) in combination with Tarceva (Erlotinib) in patients with Met diagnostic-positive non-small cell lung cancer who have received chemotherapy for advanced or metastatic disease (MetLung). Available from: http://www.clinicaltrials.gov/ct2/show/NCT01456325. NLM identifier: NCT NCT01456325. Accessed July 3, 2014.
Inman S. Onartuzumab Falters in late-stage NSCLC. Available from: http://www.onclive.com. Accessed July 11, 2014.
Spigel DR, Edelman MJ, O'Byrne K, et al. Onartuzumab plus erlotinib versus erlotinib in previously treated stage IIIb or IV NSCLC: Results from the pivotal phase III randomized, multicenter, placebo-controlled METLung (OAM4971g) global trial. J Clin Oncol. 2014;32:5s. Suppl: abstr 8000.
Genetech. A Study of Onartuzumab (MetMAb) in combination with bevacizumab (Avastin) plus platinum and paclitaxel or with pemetrexed plus platinum in patients with non-squamous non-small cell lung cancer. Available from: http://www.clinicaltrials.gov/ct2/show/NCT01496742. NLM identifier: NCT 01496742. Accessed July 3, 2014.
Genetech. A study of onartuzumab (MetMAb) versus placebo in combination with paclitaxel plus platinum in patients with squamous non-small cell lung cancer. Available from: http://www.clinicaltrials.gov/ct2/show/NCT01519804. NLM identifier: NCT 01519804. Accessed August 31, 2014.
Sadig AA, Salgia R. MET as a possible target for non-small-cell lung cancer. J Clin Oncol. 2013;31:1089–96.
Tan E, Park K, Lim WT, et al. Phase Ib study of ficlatuzumab (formerly AV-299), an anti-hepatocyte growth factor (HGF) monoclonal antibody (MAb) in combination with gefitinib (G) in Asian patients (pts) with NSCLC [abstract]. J Clin Oncol. 2011;29 Suppl:7571.
Mok T, Park K, Geater SL. A randomized phase (Ph) 2 study with exploratory biomarker analysis of ficlatuzumab (F) a humanized hepatocyte growth factor (HGF) inhibitory MAB in combination with gefitinib (G) versus G in Asian patients (pts) with lung adenocarcinoma (LA). Ann Oncol. 2012;23 Suppl 9:1198P.
Ahmad Tarhini. AMG 102 and erlotinib for advanced non-small cell lung cancer. Available from: http://www.clinicaltrials.gov/ct2/show/NCT01233687. NLM identifier: NCT NCT01233687. Accessed July 3, 2014.
Compliance with Ethics Guidelines
Conflict of InterestJoanna Goździk-Spychalska, Katarzyna Szyszka-Barth, Łukasz Spychalski, Katarzyna Ramlau, Jerzy Wójtowicz, Halina Batura-Gabryel, and Rodryg Ramlau declare that they have no conflict of interest..
Human and Animal Rights and Informed ConsentThis article does not contain any studies with human or animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Goździk-Spychalska, J., Szyszka-Barth, K., Spychalski, Ł. et al. c-MET Inhibitors in the Treatment of Lung Cancer. Curr. Treat. Options in Oncol. 15, 670–682 (2014). https://doi.org/10.1007/s11864-014-0313-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11864-014-0313-5