Abstract
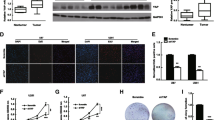
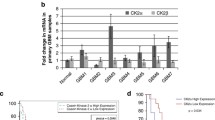
Identification of molecular pathways that are essential for cancer cell survival is vital for understanding the underlying biology, as well as to design effective cancer therapeutics. β-catenin, a multifunctional oncogenic protein, participates in cell development. Its multifaceted functions primarily lie to the subcellular distribution. The present study demonstrated that β-catenin accumulated in the nucleus to a greater extent in high-grade gliomas compared with low-grade gliomas. In addition, nuclear localization correlated with a worse prognosis for patients, as determined by immunohistochemical analysis of 74 glioma samples. Nuclear expression of β-catenin was down-regulated in LN229 and U87 glioma cells by a small molecule inhibitor of β-catenin/TCF4 signaling, demonstrating strongly inhibited β-catenin/TCF4 transcriptional activity and STAT3 luciferase activity, as well as decreased mRNA and protein levels of nuclear β-catenin, TCF4, EGFR, AKT1, AKT2 and STAT3. Furthermore, repressed nuclear translocation of β-catenin resulted in inhibition of proliferation and invasiveness, and also induced apoptosis of glioma cells. Similar results were also observed in vivo; intratumoral injection of such small molecule inhibitor downregulated expression of nuclear β-catenin, TCF4, and components of the EGFR pathway, and also delayed tumor growth in nude mice harboring subcutaneous U87 xenografts. Results from the present study provided evidence that nuclear accumulation of β-catenin participated in malignant progression of gliomas and implicated poor prognosis, highlighting it as a potential therapeutic target for gliomas.





Similar content being viewed by others
References
Adamson C, Kanu OO, Mehta AI, Di C, Lin N, Mattox AK, Bigner DD (2009) Glioblastoma multiforme: a review of where we have been and where we are going. Expert Opin Investig Drugs 18(8):1061–1083
Armanious H, Gelebart P, Mackey J, Ma Y, Lai R (2010) STAT3 upregulates the protein expression and transcriptional activity of beta-catenin in breast cancer. Int J Clin Exp Pathol 3(7):654–664
Battaglia S, Maguire O, Campbell MJ (2010) Transcription factor co-repressors in cancer biology: roles and targeting. Int J Cancer 126(11):2511–2519
Boras-Granic K, Wysolmerski JJ (2008) Wnt signaling in breast organogenesis. Organogenesis 4(2):116–122
Brocardo M, Henderson BR (2008) Detection of cytoplasmic and nuclear localization of adenomatous polyposis coli (APC) protein in cells. Methods Mol Biol 468:77–89
Cadigan KM, Peifer M (2009) Wnt signaling from development to disease: insights from model systems. Cold Spring Harb Perspect Biol 1(2):a002881
Chen L, Huang K, Han L, Shi Z, Zhang K, Pu P, Jiang C, Kang C (2011) beta-catenin/Tcf-4 complex transcriptionally regulates AKT1 in glioma. Int J Oncol 39(4):883–890
Eleftheriou A, Yoshida M, Henderson BR (2001) Nuclear export of human beta-catenin can occur independent of CRM1 and the adenomatous polyposis coli tumor suppressor. J Biol Chem 276(28):25883–25888
Handeli S, Simon JA (2008) A small-molecule inhibitor of Tcf/beta-catenin signaling down-regulates PPARgamma and PPARdelta activities. Mol Cancer Ther 7:521–529
Henderson BR (2000) Nuclear-cytoplasmic shuttling of APC regulates beta-catenin subcellular localization and turnover. Nat Cell Biol 2(9):653–660
Henderson LJ, Al-Harthi L (2011) Role of beta-catenin/TCF-4 signaling in HIV replication and pathogenesis: insights to informing novel anti-HIV molecular therapeutics. J Neuroimmune Pharmacol 6(2):247–259
Horst D, Reu S, Kriegl L, Engel J, Kirchner T, Jung A (2009) The intratumoral distribution of nuclear beta-catenin is a prognostic marker in colon cancer. Cancer 115(10):2063–2070
Hu T, Li C (2010) Convergence between Wnt-beta-catenin and EGFR signaling in cancer. Mol Cancer 9:236
Huang K, Zhang JX, Han L, You YP, Jiang T, Pu PY, Kang CS (2010) MicroRNA roles in beta-catenin pathway. Mol Cancer 9:252
Jordan BA, Kreutz MR (2009) Nucleocytoplasmic protein shuttling: the direct route in synapse-to-nucleus signaling. Trends Neurosci 32(7):392–401
Kanwar SS, Yu Y, Nautiyal J, Patel BB, Majumdar AP (2010) The Wnt/beta-catenin pathway regulates growth and maintenance of colonospheres. Mol Cancer 9:212
Kawada M, Seno H, Uenoyama Y, Sawabu T, Kanda N, Fukui H, Shimahara Y, Chiba T (2006) Signal transducers and activators of transcription 3 activation is involved in nuclear accumulation of beta-catenin in colorectal cancer. Cancer Res 66(6):2913–2917
Krieghoff E, Behrens J, Mayr B (2006) Nucleo-cytoplasmicdistribution of beta-catenin is regulated by retention. J Cell Sci 119(7):1453–1463
Miravet S, Piedra J, Miro F, Itarte E, Garcia de Herreros A, Dunach M (2002) The transcriptional factor Tcf-4 contains different binding sites for beta-catenin and plakoglobin. J Biol Chem 277(3):1884–1891
Phelps RA, Chidester S, Dehghanizadeh S, Phelps J, Sandoval IT, Rai K, Broadbent T, Sarkar S, Burt RW, Jones DA (2009) A two-step model for colon adenoma initiation and progression caused by APC loss. Cell 137(4):623–634
Prasad CP, Gupta SD, Rath G, Ralhan R (2007) Wnt signaling pathway in invasive ductal carcinoma of the breast: relationship between beta-catenin, dishevelled and cyclin D1 expression. Oncology 73(1–2):112–117
Rossi M, Magnoni L, Miracco C, Mori E, Tosi P, Pirtoli L, Tini P, Oliveri G, Cosci E, Bakker A (2011) β-catenin and Gli1 are prognostic markers in glioblastoma. Cancer Biol Ther 11(9):753–769
Schmitz Y, Wolkenhauer O, Rateitschak K (2011) Nucleo-cytoplasmic shuttling of APC can maximize beta-catenin/TCF concentration. J Theor Biol 279(1):132–142
Stenner M, Yosef B, Huebbers CU, Preuss SF, Dienes HP, Speel EJ, Odenthal M, Klussmann JP (2011) Nuclear translocation of beta-catenin and decreased expression of epithelial cadherin in human papillomavirus-positive tonsillar cancer: an early event in human papillomavirus-related tumour progression? Histopathology 58(7):1117–1126
Sugioka K, Mizumoto K, Sawa H (2011) Wnt regulates spindle asymmetry to generate asymmetric nuclear beta-catenin in C. elegans. Cell 146(6):942–954
Wang Y, Chen L, Bao Z, Li S, You G, Yan W, Shi Z, Liu Y, Yang P, Zhang W, Han L, Kang C, Jiang T (2011) Inhibition of STAT3 reverses alkylator resistance through modulation of the AKT and beta-catenin signaling pathways. Oncol Rep 26(5):1173–1180
Wiechens N, Fagotto F (2001) CRM1- and Ran-independent nuclear export of beta-catenin. Curr Biol 11(1):18–27
Yue X, Lan F, Yang W, Yang Y, Han L, Zhang A, Liu J, Zeng H, Jiang T, Pu P, Kang C (2010) Interruption of beta-catenin suppresses the EGFR pathway by blocking multiple oncogenic targets in human glioma cells. Brain Res 1366:27–37
Zhang CZ, Zhang JX, Zhang AL, Shi ZD, Han L, Jia ZF, Yang WD, Wang GX, Jiang T, You YP, Pu PY, Cheng JQ, Kang CS (2010a) MiR-221 and miR-222 target PUMA to induce cell survival in glioblastoma. Mol Cancer 9:229
Zhang J, Han L, Zhang A, Wang Y, Yue X, You Y, Pu P, Kang C (2010b) AKT2 expression is associated with glioma malignant progression and required for cell survival and invasion. Oncol Rep 24(1):65–72
Zhang J, Huang K, Shi Z, Zou J, Wang Y, Jia Z, Zhang A, Han L, Yue X, Liu N, Jiang T, You Y, Pu P, Kang C (2011a) High beta-catenin/Tcf-4 activity confers glioma progression via direct regulation of AKT2 gene expression. Neuro Oncol 13(6):600–609
Zhang N, Wei P, Gong A, Chiu WT, Lee HT, Colman H, Huang H, Xue J, Liu M, Wang Y, Sawaya R, Xie K, Yung WK, Medema RH, He X, Huang S (2011b) FoxM1 promotes beta-catenin nuclear localization and controls Wnt target-gene expression and glioma tumorigenesis. Cancer Cell 20(4):427–442
Zilman A, Di Talia S, Chait BT, Rout MP, Magnasco MO (2007) Efficiency, selectivity, and robustness of nucleocytoplasmic transport. PLoS Comput Biol 3(7):e125
Acknowledgments
This work was supported by China National Natural Scientific Fund (30971136, 81001128, 81172406), Tianjin Science and Technology Committee (09JCZDJC17600, 10SYSYJC28800), National High Technology Research and Development Program 863(2012AA02A508). The authors report no conflict of interest concerning materials or methods used in this study, or findings specified in this paper.
Author information
Authors and Affiliations
Corresponding author
Additional information
Zhendong Shi, Xiaomin Qian and Lanquan Li contributed equally to this work
Rights and permissions
About this article
Cite this article
Shi, Z., Qian, X., Li, L. et al. Nuclear Translocation of β-catenin is Essential for Glioma Cell Survival. J Neuroimmune Pharmacol 7, 892–903 (2012). https://doi.org/10.1007/s11481-012-9354-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11481-012-9354-3