Abstract
Structural, molecular and biochemical approaches have contributed to piecing together the puzzle of how matrix metalloproteinases (MMPs) work and contribute to various disease processes. However, MMPs have many unexpected substrates other than components of the extracellular matrix which profoundly influence cell behaviour, survival and death. With the current understanding of diverse/novel roles of matrix metalloproteinases—particularly their direct or indirect relevance for the early steps during programmed cell death—some seemingly contrasting results seem less surprising. To better target MMPs an appreciation of their many extracellular, intracellular and intranuclear functions, often acting in opposing directions with paradoxical roles in cell death, is carefully required.
Similar content being viewed by others
References
Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer 2002; 2: 163–176.
Vu TH, Werb Z. Matrix metalloproteinases: Effectors of development and normal physiology. Genes Dev 2000; 14: 2123–2133.
Vu TH. Don’t mess with the matrix. Nat Genet 2001; 28: 202–203.
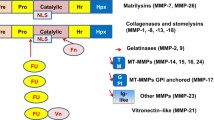
Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: Structure, function and biochemistry. Circ Res 2003; 92: 827–839.
Green DR, Evan GI. A matter of life and death. Cancer Cell 2002; 1: 19–30.
Cryns V, Yuan J. Proteases to die for. Genes Develop 1998; 12: 1551–1570.
Luchetti F, Mariani AR, Columbaro M, Di Baldassarre A, Cinti C, Falcieri E. Apoptotic pathways depend on the target enzymatic activity and not on the triggering agent. Scanning 1999; 21: 29–35.
Abraham MC, Shaham S. Death without caspases, caspases without death. Trends Cell Biol 2004; 14: 184–193.
Meier P, Silke J. Programmed cell death: Superman meets Dr Death. Nat Cell Biol 2003; 5: 1035–1038.
Lockshin RA, Zakeri Z. Caspase-independent cell death? Oncogene 2004; 23: 2766–2773.
Mannello F, Gazzanelli G. Tissue inhibitors of metalloproteinases and programmed cell death: Conundrums, controversies and potential implications. Apoptosis 2001; 6: 479–482.
Lynch CC, Matrisian LM. Matrix metalloproteinases in tumour-host cell communication. Differentiation 2002; 70: 561–573.
Brinckerhoff CE, Matrisian LM. Matrix metalloproteinases: A tail of a frog that became a prince. Nat Rev Mol Cell Biol 2002; 3: 207–214.
Online link to revised bioactive MMP substrates at: http://www.clip.ubc.ca/mmp.shtm
Lee R, Kermani P, Teng KK, Hempstead BL. Regulation of cell survival by secreted proneurotrophins. Science 2001; 294: 1945–1948.
Vaillant C, Meissirel C, Mutin M, Belin F, Lund LR, Thomasset N. MMP-9 deficiency affects axonal outgrowth, migration, and apoptosis in the developing cerebellum. Mol Cell Neurosci 2003; 24: 395–408.
Chintala SK, Zhang X, Austin JS, Fini ME. Deficiency in matrix metalloproteinase B (MMP-9) protects against retinal ganglion cell death after optic nerve ligation. J Biol Chem 2002; 277: 47461–47468.
Lee SR, Lo EH. Induction of caspase-mediated cell death by metalloproteinases in cerebral endothelial cells after hypoxia-reoxygeneration. J Cereb Blood Flow Metab 2004; 24: 720–727.
Wee Yong V, Power C, Forsyth P, Edwards DR. Metalloproteinases in biology and pathology of the nervous system. Nat Rev Neurosci 2001; 2: 502–511.
Zhang K, McQuibban GA, Silva C, et al. HIV-induced metalloproteinase processing of the chemokine stromal cell derived factor-1 causes neurodegeneration. Nat Neurosci 2003; 6: 1009–1011.
Schönbeck U, Mach F, Libby P. Generation of biologically active IL-1β by matrix metalloproteinases: A novel caspase-1-independent pathway of IL-1β processing. J Immunol 1998; 161: 3340–3346.
Dinarello CA. Interleukin-1, interleukin-1 receptors and interleukin-1 receptor antagonist. Int Rev Immunol 1998; 16: 457–499.
Maquart FX, Pasco S, Ramont L, Hornebeck W, Monboisse JC. An introduction to matrikines: Extracellular matrix-derived peptides which regulate cell activity. Implication in tumor invasion. Crit Rev Oncol Hematol 2004; 49: 199–202.
Bellon G, Martiny L, Robinet A. Matrix metalloproteinases and matrikines in angiogenesis. Crit Rev Oncol Hematol 2003; 49: 203–220.
Wang X, Lee SR, Arai K, et al. Lipoprotein receptor-mediated induction of matrix metalloproteinase by tissue plasminogen activator. Nat Med 2003; 9: 1313–1317.
Makarova A, Mikhailenko I, Bugge TH, List K, Lawrence DA, Strickland DK. The low density lipoprotein receptor-related protein modulates protease activity in the brain by mediating the cellular internalization of both neuroserpin and neuroserpin-tissue-type plasminogen activator complexes. J Biol Chem 2003; 278: 50250–50258.
Stricker TP, Dumin JA, Dickeson SK, et al. Structural analysis of the α2-integrin I domain/procollagenase-1 (matrix metalloproteinase-1) interaction. J Biol Chem 2001; 276: 29375–29381.
Conant K, St. Hillaire C, Nagase H, et al. Matrix metalloproteinase-1 interacts with neuronal integrins and stimulates dephosphorylation of Akt. J Biol Chem 2004; 279: 8056–8062.
Meerovitch K, Bergeron F, Leblond L, et al. A novel RGD antagonist that targets both αvβ3 and α5β 1 induces apoptosis of angiogenic endothelial cells on type I collagen. Vascul Pharmacol 2003; 40: 77–89.
Vos CM, Sjulson L, Nath A, et al. Cytotoxicity by matrix metalloproteinase-1 in organotypic spinal cord dissociated neuronal cultures. Exp Neurol 2000; 163: 324–330.
Cossins JA, Clements JM, Ford J, et al. Enhanced expression of MMP-7 and MMP-9 in demyelinating multiple sclerosis lesions. Acta Neuropathol 1997; 94: 590–598.
Gu Z, Kaul M, Yan B, et al. S-nitrosylation of matrix metalloproteinases: Signalling pathway to neuronal cell death. Science 2002; 297: 1186–1190.
Sternlicht MD, Werb Z. How matrix metalloproteinases regulate cell behaviour. Annu Rev Cell Dev Biol 2001; 17: 463–516.
Strand S, Vollmer P, van de Abeelen L, et al. Cleavage of CD95 by matrix metalloproteinase-7 induces apoptosis resistance in tumor cells. Oncogene 2004; 23: 3732–3736.
Wetzel M, Tibbitts J, Rosenberg GA, Cunningham LA. Vulnerability of mouse cortical neurons to doxorubicin-induced apoptosis is strain-dependent and is correlated with mRNAs encoding Fas, Fas-Ligand, and metalloproteinases. Apoptosis 2004; 9: 649–656.
Ethell DW, Kinloch R, Green DR. Metalloproteinase shedding of Fas ligand regulates β-amyloid neurotoxicity. Curr Biol 2002; 12: 1595–1600.
Powell WC, Fingleton B, Wilson CL, Boothby M, Matrisian LM. The metalloproteinase Matrilysin (MMP-7) proteolytically generates active soluble Fas ligand and potentiates epithelial cell apoptosis. Curr Biol 1999; 9: 1441–1447.
Mitsiades N, Yu WH, Poulaki V, Tsokos M, Stamenkovic I. Matrix metalloproteinase-7-mediated cleavage of Fas ligand protects tumour cells from chemotherapeutic drug cytotoxicity. Cancer Res 2001; 61: 577–581.
Fingleton B, Vargo T, Crawford HC, Matrisian LM. Matrilysin (MMP-7) expression selects for cells with reduced sensitivity to apoptosis. Neoplasia 2001; 3: 459–468.
Yu WH, Woessner JF Jr, McNeish JD, Stamenkovic I. CD44 anchors the assembly of matrilysin/MMP-7 with heparin-binding epidermal growth factor precursor and ErbB4 and regulates female reproductive organ remodelling. Genes Dev 2002; 16: 307–323.
Zahir N, Weaver VM. Death in the third dimension: Apoptosis regulation and tissue architecture. Curr Opin Genet Dev 2004; 14: 71–80.
Frisch SM, Screaton RA. Anoikis mechanisms. Curr Opin Cell Biol 2001; 13: 555–562.
Zhan M, Zhao H, Han ZC. Signalling mechanism of anoikis. Histol Histopathol 2004; 19: 973–983.
Herren B, Levkau B, Rianes EW, Ross R. Cleavage of β-catenin and plakoglobin and shedding of VE-cadherin during endothelial apoptosis: Evidence for a role for caspases and metalloproteinases. Mol Biol Cell 1998; 9: 1589–1601.
Steinhusen U, Weiske J, Badock V, Tauber R, Bommert K, Huber O. Cleavage and shedding of E-cadherin after induction of apoptosis. J Biol Chem 2001; 276: 4972–4980.
Levkau B, Kenagy RD, Karsan A, et al. Activation of metalloproteinases and their association with integrins: An auxillary apoptotic pathway in human endothelial cells. Cell Death Differ 2002; 9: 1360–1367.
Luchetti F, Mannello F, Canonico B, et al. Integrin and cytoskeleton behaviour in human neuroblastoma cells during hyperthermia-related apoptosis. Apoptosis 2004; 9: 1–14.
Wu E, Mari BP, Wang F, Anderson IC, Sunday ME, Shipp MA. Stromelysin-3 suppresses tumour cell apoptosis in a murine model. J Cell Biochem 2001; 82: 549–555.
Boulay A, Masson R, Chenard MP, et al. High cancer cell death in syngeneic tumours developed in host mice deficient for the stromelysin-3 matrix metalloproteinase. Cancer Res 2001; 61: 2189–2193.
Baserga R. The contradictions of the insulin-like growth factor 1 receptor. Oncogene 2000; 19: 5574–5581.
Ishizuya-Oka A, Li Q, Amano T, Damjanovski S, Ueda S, Shi YB. Requirement for matrix metalloproteinase stromelysin-3 in cell migration and apoptosis during tissue remodelling in Xenopus laevis. J Cell Biol 2000; 150: 1177–1188.
Sympson CJ, Talhouk RS, Alexander CM, et al. Targeted expression of stromelysin-1 in mammary gland provides evidence for a role of proteinases in branching morphogenesis and the requirement for an intact basement membrane for tissue-specific gene expression. J Cell Biol 1994; 125: 681–693.
Witty JP, Lempka T, Coffey RJ Jr, Matrisian LM. Decreased tumour formation in 7,12-dimethylbenzanthracene-treated stromelysin-1 transgenic mice is associated with alterations in mammary epithelial cell apoptosis. Cancer Res 1995; 55: 1401–1406.
Vu TH, Shipley JM, Bergers G, et al. MMP-9/gelatinase B is a key regulator of growth plate angiogenesis and apoptosis of hypertrophic chondrocytes. Cell 1998; 93: 411–422.
Bergers G, Brekken R, McMahon G, et al. Matrix metalloproteinase-9 triggers the angiogenic switch during carcinogenesis. Nat Cell Biol 2000; 2: 737–744.
Gazzanelli G, Luchetti F, Burattini S, Mannello F, Falcieri E, Papa S. Matrix metalloproteinases expression in HL-60 promyelocytic leukaemia cell during apoptosis. Apoptosis 2000; 5: 165–172.
McQuibban GA, Gong JH, Tam EM, McCulloch CA, Clark-Lewis I, Overall CM. Inflammation dampened by gelatinase A cleavage of monocyte chemoattractant protein-3. Science 2000; 289: 1202–1206.
Sheu BC, Hsu SM, Ho HN, Lien HC, Huang SC, Lin RH. A novel role of metalloproteinase in cancer-mediated immunosuppression. Cancer Res 2001; 61: 237–242.
Tamura F, Nakagawa R, Akuta T, et al. Proapoptotic effect of proteolytic activation of matrix metalloproteinases by streptococcus pyogenes thiol proteinase (Streptococcus pyrogenic exotoxin B). Infect Immun 2004; 72: 4836–4847.
Melino G. The meaning of death. Cell Death Differ 2002; 9: 347–348.
Schreader BA, Nambu JR. A fine balance for life and deth decisions. Nat Struct Mol Biol 2004; 11: 386–388.
Kwan JA, Schulze CJ, Wang W, et al. Matrix metalloproteinase-2 (MMP-2) is present in the nucleus of cardiac myocytes and is capable of cleaving poly (ADP-ribose) polymerase (PARP) in vitro. FASEB J 2004; 18: 690–692.
McCawley LJ, Matrisian LM. Matrix metalloproteinases: They’re not just for matrix anymore! Curr Opin Cell Biol 2001; 13: 534–540.
Scovassi I, Diederich M. Modulation of ploy(ADP-ribosylation) in apoptotic cells. Biochem Pharmacol 2004; 68: 1041–1047.
Martelli AM, Falcieri E, Zweyer M, et al. The controversial nuclear matrix: A balanced point of view. Histol Histopathol 2002; 17: 1193–1205.
Ritter LM, Garfield SH, Thorgeirsson UP. Tissue inhibitor of metalloproteinase-1 (TIMP-1) binds to the cell surface and translocates to the nucleus of human MCF-7 breast carcinoma cells. Biochem Biophys Res Commun 1999; 257: 494–499.
Si-Yayeb K, Monvoisin A, Mazzocco C, Lepreux S, Rosenbaum J. Unexpected localization of the matrix metalloproteinase-3 (MMP3) within the cell nucleus in liver cancer cells. J Hepathol 2003; 38: 353.
Soldani C, Scovassi AI. Poly(ADP-ribose) polymerase-1 cleavage during apoptosis: An update. Apoptosis 2002; 7: 321–328.
Brown PD. Ongoing trials with matrix metalloproteinase inhibitors. Expert Opin Investig Drugs 2000; 9: 2167–2177.
Mitsiades N, Poulaki V, Mitsiades CS, Anderson KC. Induction of tumour cell apoptosis by matrix metalloproteinases inhibitors: New tricks from a (not so) old drug. Expert Opin Investig Drugs 2001; 10: 1075–1084.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mannello, F., Luchetti, F., Falcieri, E. et al. Multiple roles of matrix metalloproteinases during apoptosis. Apoptosis 10, 19–24 (2005). https://doi.org/10.1007/s10495-005-6058-7
Issue Date:
DOI: https://doi.org/10.1007/s10495-005-6058-7