Abstract
Purpose
Despite the numerous studies on the factors involved in the genesis and growth of uterine leiomyomas, the pathogenesis of these tumors remains unknown. Intrinsic abnormalities of the myometrium, abnormal myometrial receptors for estrogen, and hormonal changes or altered responses to ischemic damage during the menstrual period may be responsible for the initiation of (epi)genetic changes found in these tumors. Considering these elements, we aimed to offer an overview about epigenetic and genetic landscape of uterine leiomyomas.
Methods
Narrative overview, synthesizing the findings of literature retrieved from searches of computerized databases.
Results
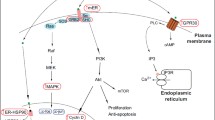
Several studies showed that leiomyomas have a monoclonal origin. Accumulating evidence converges on the risk factors and mechanisms of tumorigenesis: the translocation t (12;14) and deletion of 7q were found in the highest percentages of recurrence; dysregulation of the HMGA2 gene has been mapped within the critical 12q14–q15 locus. Estrogen and progesterone are recognized as promoters of tumor growth, and the potential role of environmental estrogens has been poorly explored. The growth factors with mitogenic activity, such as transforming growth factor-β3, fibroblast growth factor, epidermal growth factor, and insulin-like growth factor-I are elevated in fibroids and may have a role as effectors of the tumor promotion.
Conclusion
The new clues on genetics and epigenetics, as well as about the growth factors that control normal and pathological myometrial cellular biology may be of great help for the development of new effective and less invasive therapeutic strategies in the near future.

Similar content being viewed by others
References
Cardozo ER, Clark AD, Banks NK, Henne MB, Stegmann BJ, Segars JH (2012) The estimated annual cost of uterine leiomyomata in the United States. Am J Obstet Gynecol 206:211.e1–211.e9. doi:10.1016/j.ajog.2011.12.002
Laughlin SK, Schroeder JC, Baird DD (2010) New directions in the epidemiology of uterine fibroids. Semin Reprod Med 28:2014–2017. doi:10.1055/s-0030-1251477
Baird DD, Dunson DB, Hill MC, Cousins D, Schectman JM (2003) High cumulative incidence uterine leiomyoma black white woman: ultrasound evidence. Am J Obstet Gynecol 188:100–107. doi:10.1067/mob.2003.99
Tinelli A, Mynbaev OA, Sparic R, Vergara D, Di Tommaso S, Salzet M, Maffia M, Malvasi A (2016) Angiogenesis and vascularization of uterine leiomyoma: clinical value of pseudocapsule containing peptides and neurotransmitters. Curr Protein Pept Sci 18:129–139. doi:10.2174/1389203717666160322150338
Di Tommaso S, Massari S, Malvasi A, Vergara D, Maffia M, Greco M, Tinelli A (2015) Selective genetic analysis of myoma pseudocapsule and potential biological impact on uterine fibroid medical therapy. Expert Opin Ther Targets 19:7–12. doi:10.1517/14728222.2014.975793
Sato F, Miyake H, Nishi M, Mori M, Kudo R (2000) Early normal menstrual cycle pattern and the development of uterine leiomyomas. J Women’s Health Gend Based Med 9:299–302. doi:10.1089/152460900318489
Marshall LM, Spiegelman D, Goldman MB, Manson JE, Colditz GA, Barbieri RL, Stampfer MJ, Hunter DJ (1998) A prospective study of reproductive factors and oral contraceptive use in relation to the risk of uterine leiomyomata. Fertil Steril 70:432–439. doi:10.1016/s0015-0282(98)00208-8
Ross RK, Pike MC, Vessey MP, Bull D, Yeates D, Casagrande JT (1986) Risk factors for uterine fibroids: reduced risk associated with oral contraceptives. Br Med J (Clin Res Ed) 293:359–362. doi:10.1136/bmj.293.6543.359
Parazzini F, Negri E, La Vecchia C, Chatenoud L, Ricci E, Guarnerio P (1996) Reproductive factors and risk of uterine fibroids. Epidemiology 7:440–442. doi:10.1097/00001648-199607000-00018
Schneider J, Bradlow HL, Strain G, Levin J, Anderson K, Fishman J (1983) Effects of obesity on estradiol metabolism: decreased formation of nonuterotropic metabolites. J Clin Endocrinol Metab 56:973–978. doi:10.1210/jcem-56-5-973
Rose DP, Goldman M, Connolly JM, Strong LE (1991) High-fiber diet reduces serum estrogen concentrations in premenopausal women. Am J Clin Nutr 54:520–525
Prentice R, Thompson D, Clifford C, Gorbach S, Goldin B, Byar D (1990) Dietary fat reduction and plasma estradiol concentration in healthy postmenopausal women. The Women’s Health Trial Study Group. J Natl Cancer Inst 82:129–134. doi:10.1093/jnci/82.2.129
Ginsburg J, Prelevic GM (2000) Lack of significant hormonal effects and controlled trials of phyto-oestrogens. Lancet 355:163–164. doi:10.1016/s0140-6736(99)00428-6
Faerstein E, Szklo M, Rosenshein N (2001) Risk factors for uterine leiomyoma: a practice-based case-control study. I. African–American heritage, reproductive history, body size, and smoking. Am J Epidemiol 153:1–10. doi:10.1093/aje/153.1.1
Hudson BG, Tryggvason K, Sundaramoorthy M, Neilson EG (2003) Alport’s syndrome, Goodpasture’s syndrome, and type IV collagen. N Engl J Med 348:2543–2556. doi:10.1056/nejmra022296
Commandeur AE, Styer AK, Teixeira JM (2015) Epidemiological and genetic clues for molecular mechanism involved in uterine leiomyoma development and growth. Hum Reprod Update 21:593–615. doi:10.1093/humupd/dmv030
American College of Obstetricians and Gynecologist (2008) ACOG practice bulletin. Alternatives to hysterectomy in the management of leiomyiomas. Obstet Gynecol 112:387–400. doi:10.1097/AOG.0b013e318183fbab
Solnik MJ, Munro MG (2014) Indication and alternatives to hysterectomy. Clin Obstet Gynecol 57:14–42. doi:10.1097/grf.0000000000000010
Laganà AS, Giacobbe V, Triolo O, Granese R, Ban Frangež H, Vrtačnik-Bokal E, Ietto C, Palmara VI (2016) Dienogest as preoperative treatment of submucous myomas for hysteroscopic surgery: a prospective, randomized study. Gynecol Endocrinol 32:408–411. doi:10.3109/09513590.2015.1128409
Vitale SG, Padula F, Gulino FA (2015) Management of uterine fibroids in pregnancy: recent trends. Curr Opin Obstet Gynecol 27:432–437. doi:10.1097/GCO.0000000000000220
Polizzi S, Giuca R, Falduzzi C, Ippolito R, Vitale SG, Matarazzo MG, Bellia A, Gangarossa G, Franzò E, Cianci S, Cianci A (2011) Arterial embolization of uterine fibroids. G Ital di Ostet e Ginecol 33:163–167
Vitale SG, Giuca R, Cianci S, Matarazzo MG, Gulino FA, Giuffrida E, Cavallaro A, Panella M (2011) Management of uterine fibroma during pregnancy: a case report. Gazz Med Ital Arch Sci Med 170:201–206
Vitale SG, Laganà AS, Valenti G, La Rosa VL (2017) Comment on “laparoscopic myomectomy of a symptomatic uterine leiomyoma in a 15-year-old adolescent”. J Pediatr Adolesc Gynecol 30:442–443. doi:10.1016/j.jpag.2016.11.013
Vitale SG, Tropea A, Rossetti D, Carnelli M, Cianci A (2013) Management of uterine leiomyomas in pregnancy: review of literature. Updates Surg 65:179–182. doi:10.1007/s13304-013-0198-z
Fisher K, McDannold NJ, Tempany CM, Jolesz FA, Fennessy FM (2015) Potential of minimally invasive procedures in the treatment of uterine fibroids: a focus on magnetic resonance-guided focused ultrasound therapy. Int J Women’s Health 7:901–912. doi:10.2147/ijwh.s55564
Munro MG, Critchley HO, Broder MS, Fraser IS, FIGO Working Group on Menstrual Disorders (2011) FIGO classification system (PALM-COEIN) for causes of abnormal uterine bleeding in nongravid women of reproductive age. Int J Gynaecol Obstet 113:3–13. doi:10.1016/j.ijgo.2010.11.011
Sparic R, Mirkovic L, Malvasi A, Tinelli A (2016) Epidemiology of uterine myomas: a review. Int J Fertil Steril 9:424–435. doi:10.22074/ijfs.2015.4599
Kjerulff KH, Langenberg P, Seidman JD, Stolley PD, Guzinski GM (1996) Uterine leiomyomas. Racial differences in severity, symptoms and age at diagnosis. J Reprod Med 41:483–490
Taioli E, Bradlow HL, Garbers SV, Sepkovic DW, Osborne MP, Trachman J, Ganguly S, Garte SJ (1999) Role of estradiol metabolism and CYP1A1 polymorphisms in breast cancer risk. Cancer Detect Prev 23:2327. doi:10.1046/j.1525-1500.1999.09912.x
Friedman AJ, Harrison-Atlas D, Barbieri RL, Benacerraf B, Gleason R, Schiff I (1989) A randomized, placebo-controlled, double-blind study evaluating the efficacy of leuprolide acetate depot in the treatment of uterine leiomyomata. Fertil Steril 51:251–256. doi:10.1016/s0015-0282(16)60486-7
Rein MS, Barbieri RL, Friedman AJ (1995) Progesterone: a critical role in the pathogenesis of uterine myomas. Am J Obstet Gynecol 172:14–18. doi:10.1016/0002-9378(95)90077-2
Murphy AA, Kettel LM, Morales AJ, Roberts VJ, Yen SS (1993) Regression of uterine leiomyomata in response to the antiprogesterone RU 486. J Clin Endocrinol Metab 76:513–517. doi:10.1210/jcem.76.2.8432797
Patel A, Malik M, Britten J, Cox J, Catherino WH (2016) Mifepristone inhibits extracellular matrix formation in uterine leiomyoma. Fertil Steril 105:1102–1110. doi:10.1016/j.fertnstert.2015.12.021
Ohara N (2009) Sex steroidal modulation of collagen metabolism in uterine leiomyomas. Clin Exp Obstet Gynecol 36:10–11
Donnez J, Tatarchuk TF, Bouchard P, Puscasiu L, Zakharenko NF, Ivanova T, Ugocsai G, Mara M, Jilla MP, Bestel E, Terrill P, Osterloh I, Loumaye E, PEARL I Study Group (2012) Ulipristal acetate versus placebo for fibroid treatment before surgery. N Eng J Med 366:409–420. doi:10.1056/NEJMoa1103182
Teixeira J, Rueda BR, Pru JK (2008) Uterine stem cells. Harvard Stem Cell Institute, Cambridge. http://www.ncbi.nlm.nih.gov/books/NBK27042/. Accessed 13 Sept 2016
Ono M, Yin P, Navarro A, Moravek MB, Coon JS 5th, Druschitz SA, Serna VA, Qiang W, Brooks DC, Malpani SS, Ma J, Ercan CM, Mittal N, Monsivais D, Dyson MT, Yemelyanov A, Maruyama T, Chakravarti D, Kim JJ, Kurita T, Gottardi CJ, Bulun SE (2013) Paracrine activation of WNT/beta-catenin pathway in uterine leiomyomas stem cells promotes tumor growth. Proc Natl Acad Sci USA 110:17053–17058. doi:10.1073/pnas.1313650110
Moravek MB, Yin P, Ono M, Coon JS 5th, Dyson MT, Navarro A, Marsh EE, Chakravarti D, Kim JJ, Wei JJ, Bulun SE (2015) Ovarian steroids, stem cells and uterine leiomyoma: therapeutic implications. Hum Reprod Up 21:1–12. doi:10.1093/humupd/dmu048
Rizzello A, Franck J, Pellegrino M, De Nuccio F, Simeone P, Fiore G, Di Tommaso S, Malvasi A, Tinelli A, Fournier I, Salzet M, Maffia M, Vergara D (2016) A proteomic analysis of human uterine myoma. Curr Protein Pept Sci 18:167–174. doi:10.2174/1389203717666160322150603
Resta L (2004) Uterine myomas and histopathology. In: Tinelli A, Malvasi A (eds) Uterine myoma, myomectomy and minimally invasive treatments. Springer, New York, pp 27–38
Norris HJ, Hilliard GD, Irey NS (1988) Hemorrhagic cellular leiomyomas (“apoplectic leiomyoma”) of the uterus associated with pregnancy and oral contraceptives. Int J Gynecol Pathol 7:212–224. doi:10.1097/00004347-198809000-00002
Atkins K, Bell S, Kempson R, Hendrickson M (2001) Epithelioid smooth muscle of the uterus. Modern Pathol 14:132A
Ly A, Mills AM, McKenney JK, Balzer BL, Kempson RL, Hendrickson MR, Longacre TA (2013) Atypical leiomyomas of the uterus: a clinicopathologic study of 51 cases. Am J Surg Pathol 37:643–649. doi:10.1097/PAS.0b013e3182893f36
Bodner K, Bodner-Adler B, Kimberger O, Czerwenka K, Leodolter S, Mayerhofer K (2003) Evaluating prognostic parameters in women with uterine leiomyosarcoma. A clinicopathologic study. J Reprod Med 48:95–100
Choo KJ, Lee HJ, Lee TS, Kim JH, Koh SB, Choi YS (2015) Intrapelvic dissemination of early low-grade endometrioid stromal sarcoma due to electronic morcellation. Obstet Gynecol Sci 58:414–417. doi:10.5468/ogs.2015.58.5.414
Linder D, Gartler SM (1965) Glucose-6-phosphate dehydrogenase mosaicism: utilization as a cell marker in the study of leiomyomas. Science 150:67–69. doi:10.1126/science.150.3692.67
Hashimoto K, Azuma C, Kamiura S, Kimura T, Nobunaga T, Kanai T, Sawada M, Noguchi S, Saji F (1995) Clonal determination of uterine leiomyomas by analyzing differential inactivation of the X-chromosome-linked phosphoglycerokinase gene. Gynecol Obstet Invest 40:204–208. doi:10.1159/000292336
Canevari RA, Pontes A, Rosa FE, Rainho CA, Rogatto SR (2005) Independent clonal origin of multiple uterine leiomyomas that was determined by X chromosome inactivation and microsatellite analysis. Am J Obstet Gynecol 193:1395–1403. doi:10.1016/j.ajog.2005.02.097
Cai YR, Dia XL, Wang SF, Zhang W, Zhang HT, Su Q (2007) X-chromosomal inactivation analysis of uterine leiomyomas reveals a common clonal origin of different tumor nodules in some multiple leiomyomas. Int J Onc 31:1379–1389. doi:10.3892/ijo.31.6.1379
Holdsworth-Carson SJ, Zaitseva M, Vollenhoven BJ, Rogers PA (2014) Clonality of smooth muscle and fibroblast cell populations isolated from human fibroid and myometrial tissues. Mol Human Reprod 20:250–259. doi:10.1093/molehr/gat083
Sandberg AA (2005) Updated on the cytogenetics and molecular genetics of bone and soft tissue tumors: leiomyoma. Cancer Genet Cytogenet 158:1–26. doi:10.1016/j.cancergencyto.2004.08.025
Medikare V, Kandukuri LR, Anathapur V, Deenadayal M, Pratibbha N (2011) The genetic bases of uterine fibroids; a review. J Reprod Infertil 12:181–191
Ligon AH, Morton CC (2000) Genetics of uterine leiomyomata. Genes Chromosomes Cancer 28:235–245. doi:10.1002/1098-2264(200007)28:3<235:aid-gcc1>3.3.co;2-z
Nilbert M, Heim S (1990) Uterine leiomyoma cytogenetics. Genes Chromosomes Cancer 2:3–13. doi:10.1002/gcc.2870020103
Gattas GJ, Quade BJ, Nowak RA, Morton CC (1999) HMGIC expression in human adult and fetal tissues and in uterine leiomyomata. Genes Chromosomes Cancer 25:316–322. doi:10.1002/(sici)1098-2264(199908)25:4<316:aid-gcc2>3.3.co;2-s
Havel G, Dahlenfors R, Wedell B, Mark J (1989) Similar chromosomal evolution in a uterine stromomyosarcoma and in one of two leiomyomas from the same patient. APMIS 97:143–146. doi:10.1111/j.1699-0463.1989.tb00769.x
Pandis N, Heim S, Bardi G, Mandahl N, Mitelman F (1990) High resolution mapping of consistent leiomyoma breakpoints in chromosomes 12 and 14 to 12q15 and 14q24.1. Genes Chromosomes Cancer 2:227–230. doi:10.1002/gcc.2870020311
Nilbert M, Mandahl N, Heim S, Rydholm A, Helm G, Willén H, Baldetorp B, Mitelman F (1990) Complex karyotypic changes, including rearrangements of 12q13 and 14q24, in two leiomyosarcomas. Cancer Genet Cytogenet 48:217–223. doi:10.1016/0165-4608(90)90123-r
Nilbert M, Heim S, Mandahl N, Flodérus UM, Willén H, Mitelman F (1990) Characteristic chromosome abnormalities, including rearrangements of 6p, del(7q), +12, and t(12;14), in 44 uterine leiomyomas. Hum Genet 85:605–611. doi:10.1007/bf00193583
Pandis N, Heim S, Bardi G, Flodérus UM, Willén H, Mandahl N, Mitelman F (1991) Chromosome analysis of 96 uterine leiomyomas. Cancer Genet Cytogenet 55:11–18. doi:10.1016/0165-4608(91)90229-n
Vanni R, Van Roy N, Lecca U, Speleman F (1992) Uterine leiomyoma cytogenetics. III. Interphase cytogenetic analysis of karyo typically normal uterine leiomyoma excludes possibility of undetected trisomy 12. Cancer Genet Cytogenet 62:40–42
Prayson RA, Goldblum JR, Hart WR (1997) Epithelioid smooth-muscle tumors of the uterus: a clinicopathologic study of 18 patients. Am J Surg Pathol 21:383–391. doi:10.1097/00000478-199704000-00003
Aman P, Ron D, Mandahl N, Fioretos T, Heim S, Arheden K, Willén H, Rydholm A, Mitelman F (1992) Rearrangement of the transcription factor gene CHOP in myxoid liposarcomas with t(12;16)(q13;p11). Genes Chromosomes Cancer 5:278–285. doi:10.1002/gcc.2870050403
Schmidt LS, Linehan WM (2014) Hereditary leiomyomatosis and renal cell carcinoma. Int J Nephrol Renovasc Dis 7:253–260. doi:10.2147/ijnrd.s42097
Sanz-Ortega J, Vocke C, Stratton P, Linehan WM, Merino MJ (2013) Morphologic and molecular characteristic of uterine leiomyomas in hereditary leiomyomatosis and renal cancer (HLRCC) syndrome. Am J Surg Pathol 37:74–80. doi:10.1097/pas.0b013e31825ec16f
Wei MH, Toure O, Glenn GM, Pithukpakorn M, Neckers L, Stolle C, Choyke P, Grubb R, Middelton L, Turner ML, Walther MM, Merino MJ, Zbar B, Linehan WM, Toro JR (2006) Novel mutations in FH and expansion of the spectrum of phenotypes expressed in families with hereditary leiomyomatosis and renal cell cancer. J Med Genet 43:18–27. doi:10.1136/jmg.2005.033506
Mäkinen N, Mehine M, Tolvanen J, Kaasinen E, Li Y, Lehtonen HJ, Gentile M, Yan J, Enge M, Taipale M, Aavikko M, Katainen R, Virolainen E, Böhling T, Koski TA, Launonen V, Sjöberg J, Taipale J, Vahteristo P, Aaltonen LA (2011) MED12, the mediator complex subunit 12 gene, is mutated at high frequency in uterine leiomyomas. Science 334:252–255. doi:10.1126/science.1208930
Makinen N, Vahteristo P, Kampjiarvi K, Arola J, Butzow R, Aaltonen L (2013) MED12 exon 2 mutations in histopathological uterine leiomyoma variants. EJ Hum Genet 21:1300–1303. doi:10.1038/ejhg.2013.33
Taatjes DJ (2010) The human Mediator complex: a versatile, genome-wide regulator of transcription. Trends Biochem Sci 35:315–322. doi:10.1016/j.tibs.2010.02.004
Kim S, Xu X, Hecht A, Boyer TG (2006) Mediator is a transducer of Wnt/β-catenin signaling. J Biol Chem 281:14066–14075. doi:10.1074/jbc.m602696200
Markowski DN, Bartnitzke S, Loning T, Drieschner N, Helmke BM, Bullerdiek J (2012) MED12 mutation in uterine fibroids-their relationship to cytogenetic subgroups. Int J Cancer 131:1528–1536. doi:10.1002/ijc.27424
Bertsch E, Qiang W, Zhang Q, Espona-Fiedler M, Druschitz S, Liu Y, Mittal K, Kong B, Kurita T, Wei JJ (2014) MED12 and HMGA2 mutations: two independent genetic events in uterine leiomyoma and leiomyosarcoma. Mod Patol 27:1144–1153. doi:10.1038/modpathol.2013.243
Moon YS, Park SK, Kim HT, Lee TS, Kim JH, Choi YS (2010) Imprinting and expression status of isoforms 1 and 2 of PEG1/MEST gene in uterine leiomyoma. Gynecol Obstet Invest 70:120–125. doi:10.1159/000301555
Pérot G, Croce S, Ribeiro A, Lagarde P, Velasco V, Neuville A, Coindre JM, Stoeckle E, Floquet A, MacGrogan G, Chibon F (2012) MED12 alterations in both human and malignant uterine soft tissue tumors. PLoS ONE 7:e40015. doi:10.1371/journal.pone.0040015
Kämpjärvi K, Mäkinen N, Kilpivaara O, Arola J, Heinonen HR, Böhm J, Abdel-Wahab O, Lehtonen HJ, Pelttari LM, Mehine M, Schrewe H, Nevanlinna H, Levine RL, Hokland P, Böhling T, Mecklin JP, Bützow R, Aaltonen LA, Vahteristo P (2012) Somatic MED12 mutations in uterine leiomyosarcoma and colorectal cancer. Br J Cancer 107:1761–1765. doi:10.1038/bjc.2012.428
Matsubara A, Sekine S, Yoshida M, Yoshida A, Taniguchi H, Kushima R, Tsuda H, Kanai Y (2013) Prevalence of MED12 mutations in uterine and extrauterine smooth muscle tumours. Histopathology 62:657–661. doi:10.1111/his.12039
Christacos NC, Quade BJ, Dal Cin P, Morton CC (2006) Uterine leiomyomata with deletions of Ip represent a distinct cytogenetic subgroup with unusual histologic features. Gene Chromosomes Cancer 45:304–312. doi:10.1002/gcc.20291
Bertsch E, Qiang W, Zhang Q, Espona-Fiedler M, Druschitz S, Liu Y, Mittal K, Kong B, Kurita T, Wei JJ (2014) MED12 and HMGA2 mutation: two independent genetic events in uterine leiomyoma and leiomyosarcoma. Mod Pathol 27:1144–1153. doi:10.1038/modpathol.2013.243
Croce S, Chibon F (2015) MED12 and uterine smooth muscle oncogenesis: state of art and perspectives. Eur J Cancer 51:1603–1610. doi:10.1016/j.ejca.2015.04.023
Fusco A, Fedele M (2007) Roles of HMGA proteins in cancer. Nat Rev Cancer 7:899–910. doi:10.1038/nrc2271
Van de Ven WJM (1998) Genetic basis of uterine leiomyoma: involvement of high mobility group protein genes. Eur J Obstet Gynecol Reprod Biol 81:289–293. doi:10.1016/s0301-2115(98)00204-8
Quade BJ, Weremowicz S, Neskey DM, Vanni R, Ladd C, Dal Cin P, Morton CC (2003) Fusion transcripts involving HMGA2 are not a coomon molecular mechanism in uterine leiomyomata with rearrangements in 12q15. Cancer Res 633:1351–1358
Mehine M, Mäkinen N, Heinonen HR, Aaltonen LA, Vahteristo P (2014) Genomics of uterine leiomyomas: insights from high-throughput sequencing. Fertil Steril 102:621–629. doi:10.1016/j.fertnstert.2014.06.050
Mehine M, Kaasinen E, Mäkinen N, Katainen R, Kämpjärvi K, Pitkänen E, Heinonen HR, Bützow R, Kilpivaara O, Kuosmanen A, Ristolainen H, Gentile M, Sjöberg J, Vahteristo P, Aaltonen LA (2013) Characterization of uterine leiomyomas by whole-genome sequencing. N Engl J Med 369:43–53. doi:10.1056/NEJMoa1302736
Markowski DN, Von Ahsen I, Nezhad MH, Wosniok W, Helmke BM, Bullerdiek J (2010) HMGA2 and the p19Arf-TP53-CDKN1A axis: a delicate balance in the growth of uterine leiomyomas. Gene Chromosomes Cancer 49:661–668. doi:10.1002/gcc.20777
Mehine M, Kaasinen E, Heinonen HR, Mäkinen N, Kämpjärvi K, Sarvilinna N, Aavikko M, Vähärautio A, Pasanen A, Bützow R, Heikinheimo O, Sjöberg J, Pitkänen E, Vahteristo P, Aaltonen LA (2016) Integrated data analysis reveals uterine leiomyoma subtypes with distinct driver pathways and biomarkers. Proc Natl Acad Sci USA 113:1315–1320. doi:10.1073/pnas.1518752113
Tsibris JC, Maas S, Segars JH, Nicosia SV, Enkemann SA, O’Brien WF, Spellacy WN (2003) New potential regulators of uterine leiomyomata from DNA arrays: the ionotropic glutamate receptor GluR2. Biochem Biophys Res Commun 312:249–254. doi:10.1016/j.bbrc.2003.09.189
Wang H, Mahadevappa M, Yamamoto K, Wen Y, Chen B, Warrington JA, Polan ML (2003) Distinctive proliferative phase differences in gene expression in human myometrium and leiomyomata. Fertil Steril 80:266–276. doi:10.1016/s0015-0282(03)00730-1
Skubitz KM, Skubitz AP (2003) Differential gene expression in uterine leiomyoma. J Lab Clin Med 141:297–308. doi:10.1016/S0022-2143(03)00007-6
Ahn WS, Kim KW, Bae SM, Yoon JH, Lee JM, Namkoong SE, Kim JH, Kim CK, Lee YJ, Kim YW (2003) Targeted cellular process profiling approach for uterine leiomyoma using cDNA microarray, proteomics and gene ontology analysis. Int J Exp Pathol 84:267–279. doi:10.1111/j.0959-9673.2003.00362.x
Catherino W, Salama A, Potlog-Nahari C, Leppert P, Tsibris J, Segars J (2004) Gene expression studies in leiomyomata: new directions for research. Semin Reprod Med 22:83–90. doi:10.1055/s-2004-828614
Hoffman PJ, Milliken DB, Gregg LC, Davis RR, Gregg JP (2004) Molecular characterization of uterine fibroids and its implication for underlying mechanisms of pathogenesis. Fertil Steril 82:639–649. doi:10.1016/j.fertnstert.2004.01.047
Arslan AA, Gold LI, Mittal K, Suen TC, Belitskaya-Levy I, Tang MS, Toniolo P (2005) Gene expression studies provide clues to the pathogenesis of uterine leiomyoma: new evidence and a systematic review. Hum Reprod 20:852–863. doi:10.1093/humrep/deh698
Yang Q, Mas A, Diamond MP, Al-Hendy A (2016) The mechanism and function of epigenetics in uterine leiomyoma development. Reproductive Science 23:163–175. doi:10.1177/1933719115584449
Yamagata Y, Maekawa R, Asada H, Taketani T, Tamura I, Tamura H, Ogane J, Hattori N, Shiota K, Sugino N (2009) Aberrant DNA methylation status in human uterine leiomyoma. Mol Hum Reprod 15:259–267. doi:10.1093/molehr/gap010
Chen K, Rajewsky N (2007) The evolution of gene regulation by transcription factors and microRNAs. Nat Rev Genet 8:93–103. doi:10.1038/nrg1990
Ciarmela P, Islam MS, Reis FM, Gray PC, Bloise E, Petraglia F, Vale W, Castellucci M (2011) Growth factors and myometrium: biological effects in uterine fibroid and possible clinical implications. Human Reprod Update 17:772–790. doi:10.1093/humupd/dmr031
Lyons RM, Moses HL (1990) Transforming growth factors and the regulation of cell proliferation. Eur J Biochem 187:467–473. doi:10.1111/j.1432-1033.1990.tb15327.x
Arici A, Sozen I (2000) Transforming growth factor-beta3 is expressed at high levels in leiomyoma where it stimulates fibronectin expression and cell proliferation. Fertil Steril 73:1006–1011. doi:10.1016/s0015-0282(00)00418-0
Chegini N, Zhao Y, Williams RS, Flanders KC (1994) Human uterine tissue throughout the menstrual cycle expresses transforming growth factor-beta 1 (TGF beta 1), TGF beta 2, TGF beta 3, and TGF beta type II receptor messenger ribonucleic acid and protein and contains TGF beta 1-binding sites. Endocrinology 135:439–449. doi:10.1210/en.135.1.439
Andersen J (1998) Factors in fibroid growth. Baillieres Clin Obstet Gynaecol 12:225–243
Dixon D, He H, Haseman JK (2000) Immunohistochemical localization of growth factors and their receptors in uterine leiomyomas and matched myometrium. Environ Health Perspect 108:795–802. doi:10.2307/3454309
Mangrulkar RS, Ono M, Ishikawa M, Takashima S, Klagsbrun M, Nowak RA (1995) Isolation and characterization of heparin-binding growth factors in human leiomyomas and normal myometrium. Biol Reprod 53:636–646. doi:10.1095/biolreprod53.3.636
Anania CA, Stewart EA, Quade BJ, Hill JA, Nowak RA (1997) Expression of the fibroblast growth factor receptor in women with leiomyomas and abnormal uterine bleeding. Mol Hum Reprod 3:685–691. doi:10.1093/molehr/3.8.685
Stewart EA, Nowak RA (1996) Leiomyoma-related bleeding: a classic hypothesis updated for the molecular era. Hum Reprod Update 2:295–306. doi:10.1093/humupd/2.4.295
Yeh J, Rein M, Nowak R (1991) Presence of messenger ribonucleic acid for epidermal growth factor (EGF) and EGF receptor demonstrable in monolayer cell cultures of myometria and leiomyomata. Fertil Steril 56:997–1000. doi:10.1016/s0015-0282(16)54681-0
Nowak RA, Mora S, Diehl T, Rhoades AR, Stewart EA (1999) Prolactin is an autocrine or paracrine growth factor for human myometrial and leiomyoma cells. Gynecol Obstet Invest 48:127–132. doi:10.1159/000010154
Fayed YM, Tsibris JC, Langenberg PW, Robertson AL Jr (1989) Human uterine leiomyoma cells: binding and growth responses to epidermal growth factor, platelet-derived growth factor, and insulin. Lab Invest 60:30–37
Harrison-Woolrych ML, Sharkey AM, Charnock-Jones DS, Smith SK (1995) Localization and quantification of vascular endothelial growth factor messenger ribonucleic acid in human myometrium and leiomyomata. J Clin Endocrinol Metab 80:1853–1858. doi:10.1210/jcem.80.6.7775632
Hyder SM, Huang JC, Nawaz Z, Boettger-Tong H, Mäkelä S, Chiappetta C, Stancel GM (2000) Regulation of vascular endothelial growth factor expression by estrogens and progestins. Environ Health Perspect 108:785–790. doi:10.2307/3454307
Jonca F, Ortega N, Gleizes PE, Bertrand N, Plouet J (1997) Cell release of bioactive fibroblast growth factor 2 by exon 6-encoded sequence of vascular endothelial growth factor. J Biol Chem 272:24203–24209. doi:10.1074/jbc.272.39.24203
Vollenhoven BJ, Herington AC, Healy DL (1993) Messenger ribonucleic acid expression of the insulin-like growth factors and their binding proteins in uterine fibroids and myometrium. J Clin Endocrinol Metab 76:1106–1110. doi:10.1210/jc.76.5.1106
Segars JH, Parrott EC, Nagel JD, Guo XC, Gao X, Birnbaum LS, Pinn VW, Dixon D (2014) Proceedings from the Third National Institutes of Health International Congress on Advances in Uterine Leiomyoma Research: comprehensive review, conference summary and future recommendations. Hum Reprod Update 20:309–333. doi:10.1093/humupd/dmt058
Yu L, Moore AB, Dixon D (2010) Receptor tyrosine Kinases and their hormonal regulation in uterine leiomyomas. Semin Reprod Med 28:250–259. doi:10.1055/s-0030-1251482
Sumitani H, Shozu M, Segawa T, Murakami K, Yang HJ, Shimada K, Inoue M (2000) In situ estrogen synthesized by aromatase P450 in uterine leiomyoma cells promotes cell growth probably via an autocrine/intracrine mechanism. Endocrinology 141:3852–3861. doi:10.1210/endo.141.10.7719
Quade BJ, Wang TY, Sornberger K, Dal Cin P, Mutter GL, Morton CC (2004) Molecular pathogenesis of uterine smooth muscle tumors from transcriptional profiling. Genes Chromosomes Cancer 40:97–108. doi:10.1002/gcc.20018
Hodge JC, Morton CC (2007) Genetic heterogeneity among uterine leiomyomata: insight into malignant progression. Hum Mol Genet 16(1):R7–R13. doi:10.1093/hmg/ddm043
Mittal KR, Chen F, Wei JJ, Rijhvani K, Kurvathi R, Streck D, Dermody J, Toruner GA (2009) Molecular and immunohistochemical evidence for the origin of uterine leiomyosarcomas from associated leiomyoma and symplastic leiomyoma-like areas. Mod Pathol 22:1303–1311. doi:10.1038/modpathol.2009.96
Dastranj Tabrizi A, Ghojazadeh M, Thagizadeh Anvar H, Vahedi A, Naji S, Mostafidi E, Berenjian S (2015) Immunohistochemical profile of uterine leiomyoma with bizarre nuclei; comparison with conventional leiomyoma, smooth muscle tumors of uncertain malignant potential and leiomyorsarcoma. Adv Pharma Bull 5:683–687. doi:10.15171/apb.2015.093
Kim JH, Choi YJ, Kim DC, Lee SJ (2010) Leiomyosarcoma arising in a patient with prior mitotically active leiomyoma. J Obstet Gynaecol Res 36:187–190. doi:10.1111/j.1447-0756.2009.01117.x
Mayer A, Höckel M, Wree A, Leo C, Horn LC, Vaupel P (2008) Lack of hypoxic response in uterine leiomyomas despite severe tissue hypoxia. Cancer Res 68:4719–4726. doi:10.1158/0008-5472.CAN-07-6339
Author information
Authors and Affiliations
Contributions
ASL, DV, AF, AT: manuscript writing, VLLR, MN, AV, AMCR: literature screening and data selection, SG, OT: manuscript editing, SGV: project supervision and final approval.
Corresponding author
Ethics declarations
Funding
The work was not supported by any fund/grant.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
Informed consent is not required for this type of study.
Rights and permissions
About this article
Cite this article
Laganà, A.S., Vergara, D., Favilli, A. et al. Epigenetic and genetic landscape of uterine leiomyomas: a current view over a common gynecological disease. Arch Gynecol Obstet 296, 855–867 (2017). https://doi.org/10.1007/s00404-017-4515-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-017-4515-5