Abstract
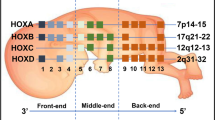
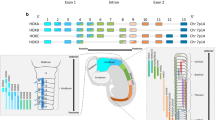
In this review, we summarize published findings on the involvement of HOX genes in oncogenesis. HOX genes are developmental genes—they code for proteins that function as critical master regulatory transcription factors during embryogenesis. Many reports have shown that the protein products of HOX genes also play key roles in the development of cancers. Based on our review of the literature, we found that the expression of HOX genes is not only up- or downregulated in most solid tumors but also that the expression of specific HOX genes in cancers tends to differ based on tissue type and tumor site. It was also observed that HOXC family gene expression is upregulated in most solid tumor types, including colon, lung, and prostate cancer. The two HOX genes that were reported to be most commonly altered in solid tumors were HOXA9 and HOXB13. HOXA were often reported to have altered expression in breast and ovarian cancers, HOXB genes in colon cancers, HOXC genes in prostate and lung cancers, and HOXD genes in colon and breast cancers. It was found that HOX genes are also regulated at the nuclear–cytoplasmic transport level in carcinomas. Tumors arising from tissue having similar embryonic origin (endodermal), including colon, prostate, and lung, showed relatively similar HOXA and HOXB family gene expression patterns compared to breast tumors arising from mammary tissue, which originates from the ectoderm. The differential expression of HOX genes in various solid tumors thus provides an opportunity to advance our understanding of cancer development and to develop new therapeutic agents.


Similar content being viewed by others
References
Gehring WJ, Hiromi Y (1986) Homeotic genes and the homeobox. Annu Rev Genet 20:147–173
Lewis EB (1978) A gene complex controlling segmentation in Drosophila. Nature 276(5688):565–570
Bridges CB (1921) Genetical and cytological proof of non-disjunction of the fourth chromosome of Drosophila melanogaster. Proc Natl Acad Sci U S A 7(7):186–192
Nourse J et al (1990) Chromosomal translocation t(1;19) results in synthesis of a homeobox fusion mRNA that codes for a potential chimeric transcription factor. Cell 60(4):535–545
Kamps MP et al (1990) A new homeobox gene contributes the DNA binding domain of the t(1;19) translocation protein in pre-B ALL. Cell 60(4):547–555
Billeter M et al (1990) Determination of the three-dimensional structure of the Antennapedia homeodomain from Drosophila in solution by 1H nuclear magnetic resonance spectroscopy. J Mol Biol 214(1):183–197
Qian YQ et al (1989) The structure of the Antennapedia homeodomain determined by NMR spectroscopy in solution: comparison with prokaryotic repressors. Cell 59(3):573–580
Scott MP (1992) Vertebrate homeobox gene nomenclature. Cell 71(4):551–553
Kanai M et al (2010) Aberrant expressions of HOX genes in colorectal and hepatocellular carcinomas. Oncol Rep 23(3):843–851
Freschi G et al (2005) Expression of HOX homeobox genes in the adult human colonic mucosa (and colorectal cancer?). Int J Mol Med 16(4):581–587
Vider BZ et al (2000) Deregulated expression of homeobox-containing genes, HOXB6, B8, C8, C9, and Cdx-1, in human colon cancer cell lines. Biochem Biophys Res Commun 272(2):513–518
Vider BZ et al (1997) Human colorectal carcinogenesis is associated with deregulation of homeobox gene expression. Biochem Biophys Res Commun 232(3):742–748
Liao WT et al (2011) HOXB7 as a prognostic factor and mediator of colorectal cancer progression. Clin Cancer Res 17(11):3569–3578
Sanz-Pamplona R et al (2011) Gene expression differences between colon and rectum tumors. Clin Cancer Res 17(23):7303–7312
Cantile M et al (2003) In vivo expression of the whole HOX gene network in human breast cancer. Eur J Cancer 39(2):257–264
Hur H et al (2014) Analysis of HOX gene expression patterns in human breast cancer. Mol Biotechnol 56(1):64–71
Makiyama K et al (2005) Aberrant expression of HOX genes in human invasive breast carcinoma. Oncol Rep 13(4):673–679
Chen H, Chung S, Sukumar S (2004) HOXA5-induced apoptosis in breast cancer cells is mediated by caspases 2 and 8. Mol Cell Biol 24(2):924–935
Raman V et al (2000) HOXA5 regulates expression of the progesterone receptor. J Biol Chem 275(34):26551–26555
Chen H et al (2007) HOXA5 acts directly downstream of retinoic acid receptor beta and contributes to retinoic acid-induced apoptosis and growth inhibition. Cancer Res 67(17):8007–8013
Morgan R et al (2012) Targeting the HOX/PBX dimer in breast cancer. Breast Cancer Res Treat 136(2):389–398
Shaoqiang C et al (2013) Expression of HOXD3 correlates with shorter survival in patients with invasive breast cancer. Clin Exp Metastasis 30(2):155–163
Shah N et al (2013) HOXB13 mediates tamoxifen resistance and invasiveness in human breast cancer by suppressing ERalpha and inducing IL-6 expression. Cancer Res 73(17):5449–5458
Ma XJ et al (2004) A two-gene expression ratio predicts clinical outcome in breast cancer patients treated with tamoxifen. Cancer Cell 5(6):607–616
Miller GJ et al (2003) Aberrant HOXC expression accompanies the malignant phenotype in human prostate. Cancer Res 63(18):5879–5888
Waltregny D et al (2002) Overexpression of the homeobox gene HOXC8 in human prostate cancer correlates with loss of tumor differentiation. Prostate 50(3):162–169
Axlund SD, Lambert JR, Nordeen SK (2010) HOXC8 inhibits androgen receptor signaling in human prostate cancer cells by inhibiting SRC-3 recruitment to direct androgen target genes. Mol Cancer Res 8(12):1643–1655
Kim SD et al (2010) HOXB13 is co-localized with androgen receptor to suppress androgen-stimulated prostate-specific antigen expression. Anat Cell Biol 43(4):284–293
Ramachandran S et al (2005) Loss of HOXC6 expression induces apoptosis in prostate cancer cells. Oncogene 24(1):188–198
Chen J et al (2013) HoxB3 promotes prostate cancer cell progression by transactivating CDCA3. Cancer Lett 330(2):217–224
Chen JL et al (2012) Deregulation of a Hox protein regulatory network spanning prostate cancer initiation and progression. Clin Cancer Res 18(16):4291–4302
Huang Q et al (2014) A prostate cancer susceptibility allele at 6q22 increases RFX6 expression by modulating HOXB13 chromatin binding. Nat Genet 46(2):126–135
Javed S and Langley SE (2013) Importance of HOX genes in normal prostate gland formation, prostate cancer development and its early detection. BJU Int
Cantile M et al (2005) cAMP induced modifications of HOX D gene expression in prostate cells allow the identification of a chromosomal area involved in vivo with neuroendocrine differentiation of human advanced prostate cancers. J Cell Physiol 205(2):202–210
Omatu T (1999) [Overexpression of human homeobox gene in lung cancer A549 cells results in enhanced motile and invasive properties]. Hokkaido Igaky Zasshi 74(5):367–376
Abe M et al (2006) Disordered expression of HOX genes in human non-small cell lung cancer. Oncol Rep 15(4):797–802
Plowright L et al (2009) HOX transcription factors are potential therapeutic targets in non-small-cell lung cancer (targeting HOX genes in lung cancer). Br J Cancer 100(3):470–475
Bodey B et al (2000) Immunocytochemical detection of homeobox B3, B4, and C6 gene product expression in lung carcinomas. Anticancer Res 20(4):2711–2716
Costa BM et al (2010) Reversing HOXA9 oncogene activation by PI3K inhibition: epigenetic mechanism and prognostic significance in human glioblastoma. Cancer Res 70(2):453–462
Murat A et al (2008) Stem cell-related “self-renewal” signature and high epidermal growth factor receptor expression associated with resistance to concomitant chemoradiotherapy in glioblastoma. J Clin Oncol 26(18):3015–3024
Gaspar N et al (2010) MGMT-independent temozolomide resistance in pediatric glioblastoma cells associated with a PI3-kinase-mediated HOX/stem cell gene signature. Cancer Res 70(22):9243–9252
Gallo M et al (2013) A tumorigenic MLL-homeobox network in human glioblastoma stem cells. Cancer Res 73(1):417–427
Tabuse M et al (2011) Functional analysis of HOXD9 in human gliomas and glioma cancer stem cells. Mol Cancer 10:60
Cantile M et al (2013) Aberrant expression of posterior HOX genes in well differentiated histotypes of thyroid cancers. Int J Mol Sci 14(11):21727–21740
Takahashi Y et al (2004) Expression profiles of 39 HOX genes in normal human adult organs and anaplastic thyroid cancer cell lines by quantitative real-time RT-PCR system. Exp Cell Res 293(1):144–153
Gendronneau G et al (2012) The loss of Hoxa5 function causes estrous acyclicity and ovarian epithelial inclusion cysts. Endocrinology 153(3):1484–1497
Cheng W et al (2005) Lineage infidelity of epithelial ovarian cancers is controlled by HOX genes that specify regional identity in the reproductive tract. Nat Med 11(5):531–537
Naora H et al (2001) Aberrant expression of homeobox gene HOXA7 is associated with mullerian-like differentiation of epithelial ovarian tumors and the generation of a specific autologous antibody response. Proc Natl Acad Sci U S A 98(26):15209–15214
Yamashita T et al (2006) Suppression of invasive characteristics by antisense introduction of overexpressed HOX genes in ovarian cancer cells. Int J Oncol 28(4):931–938
Ota T et al (2009) Expression and function of HOXA genes in normal and neoplastic ovarian epithelial cells. Differentiation 77(2):162–171
Klausen C, Leung PC, Auersperg N (2009) Cell motility and spreading are suppressed by HOXA4 in ovarian cancer cells: possible involvement of beta1 integrin. Mol Cancer Res 7(9):1425–1437
Davidson B et al (2011) Gene expression signatures differentiate ovarian/peritoneal serous carcinoma from breast carcinoma in effusions. J Cell Mol Med 15(3):535–544
Cantile M et al (2003) Hyperexpression of locus C genes in the HOX network is strongly associated in vivo with human bladder transitional cell carcinomas. Oncogene 22(41):6462–6468
Marra L et al (2013) Deregulation of HOX B13 expression in urinary bladder cancer progression. Curr Med Chem 20(6):833–839
Kim YJ et al (2013) HOXA9, ISL1 and ALDH1A3 methylation patterns as prognostic markers for nonmuscle invasive bladder cancer: array-based DNA methylation and expression profiling. Int J Cancer 133(5):1135–1142
Cantile M et al (2011) Expression of lumbosacral HOX genes, crucial in kidney organogenesis, is systematically deregulated in clear cell kidney cancers. Anticancer Drugs 22(5):392–401
Cantile M et al (2012) Increased HOX C13 expression in metastatic melanoma progression. J Transl Med 10:91
Yekta S, Tabin CJ, Bartel DP (2008) MicroRNAs in the Hox network: an apparent link to posterior prevalence. Nat Rev Genet 9(10):789–796
Niinuma T et al (2012) Upregulation of miR-196a and HOTAIR drive malignant character in gastrointestinal stromal tumors. Cancer Res 72(5):1126–1136
Nakayama I et al (2013) Loss of HOXD10 expression induced by upregulation of miR-10b accelerates the migration and invasion activities of ovarian cancer cells. Int J Oncol 43(1):63–71
Li Q, Zhu F, Chen P (2012) miR-7 and miR-218 epigenetically control tumor suppressor genes RASSF1A and Claudin-6 by targeting HoxB3 in breast cancer. Biochem Biophys Res Commun 424(1):28–33
Sun L et al (2011) MicroRNA-10b induces glioma cell invasion by modulating MMP-14 and uPAR expression via HOXD10. Brain Res 1389:9–18
Mueller DW, Bosserhoff AK (2011) MicroRNA miR-196a controls melanoma-associated genes by regulating HOX-C8 expression. Int J Cancer 129(5):1064–1074
Severino P et al (2013) MicroRNA expression profile in head and neck cancer: HOX-cluster embedded microRNA-196a and microRNA-10b dysregulation implicated in cell proliferation. BMC Cancer 13:533
Zhang JX et al (2013) HOTAIR, a cell cycle-associated long noncoding RNA and a strong predictor of survival, is preferentially expressed in classical and mesenchymal glioma. Neuro Oncol 15(12):1595–1603
Betschinger J, Knoblich JA (2004) Dare to be different: asymmetric cell division in Drosophila, C. elegans and vertebrates. Curr Biol 14(16):R674–R685
Amsellem S et al (2003) Ex vivo expansion of human hematopoietic stem cells by direct delivery of the HOXB4 homeoprotein. Nat Med 9(11):1423–1427
Sauvageau G et al (1995) Overexpression of HOXB4 in hematopoietic cells causes the selective expansion of more primitive populations in vitro and in vivo. Genes Dev 9(14):1753–1765
Buske C et al (2002) Deregulated expression of HOXB4 enhances the primitive growth activity of human hematopoietic cells. Blood 100(3):862–868
Boman BM, Wicha MS (2008) Cancer stem cells: a step toward the cure. J Clin Oncol 26(17):2795–2799
Boman BM et al (2008) How dysregulated colonic crypt dynamics cause stem cell overpopulation and initiate colon cancer. Cancer Res 68(9):3304–3313
Boman BM, Huang E (2008) Human colon cancer stem cells: a new paradigm in gastrointestinal oncology. J Clin Oncol 26(17):2828–2838
Chen YC et al (2009) Aldehyde dehydrogenase 1 is a putative marker for cancer stem cells in head and neck squamous cancer. Biochem Biophys Res Commun 385(3):307–313
Bhatlekar S et al (2014) Identification of a developmental gene expression signature, including HOX genes, for the normal human colonic crypt stem cell niche: overexpression of the signature parallels stem cell overpopulation during colon tumorigenesis. Stem Cells Dev 23(2):167–179
Vinnitsky VB (1993) Oncogerminative hypothesis of tumor formation. Med Hypotheses 40(1):19–27
Schmitt T, Ogris C, Sonnhammer EL (2014) FunCoup 3.0: database of genome-wide functional coupling networks. Nucleic Acids Res 42(Database issue):D380–D388
Magnani L et al (2011) PBX1 genomic pioneer function drives ERalpha signaling underlying progression in breast cancer. PLoS Genet 7(11):e1002368
Mo ML et al (2013) Detection of E2A-PBX1 fusion transcripts in human non-small-cell lung cancer. J Exp Clin Cancer Res 32:29
Lawrence HJ et al (1999) Frequent co-expression of the HOXA9 and MEIS1 homeobox genes in human myeloid leukemias. Leukemia 13(12):1993–1999
Kloetzli JM et al (2001) The winged helix gene, Foxb1, controls development of mammary glands and regions of the CNS that regulate the milk-ejection reflex. Genesis 29(2):60–71
Siu MK et al (2013) Stem cell transcription factor NANOG controls cell migration and invasion via dysregulation of E-cadherin and FoxJ1 and contributes to adverse clinical outcome in ovarian cancers. Oncogene 32(30):3500–3509
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bhatlekar, S., Fields, J.Z. & Boman, B.M. HOX genes and their role in the development of human cancers. J Mol Med 92, 811–823 (2014). https://doi.org/10.1007/s00109-014-1181-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-014-1181-y